当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chirality and Electronic Structure of the Thiolate-Protected Au38Nanocluster
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-06-16 , DOI: 10.1021/ja102934q Olga Lopez-Acevedo 1 , Hironori Tsunoyama 1 , Tatsuya Tsukuda 1 , Hannu Häkkinen 1 , Christine M. Aikens 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-06-16 , DOI: 10.1021/ja102934q Olga Lopez-Acevedo 1 , Hironori Tsunoyama 1 , Tatsuya Tsukuda 1 , Hannu Häkkinen 1 , Christine M. Aikens 1
Affiliation
|
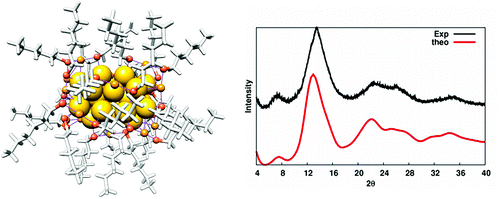
Structural, electronic, and optical properties of the thiolate-protected Au(38)(SR)(24) cluster are studied by density-functional theory computations (R = CH(3) and R = C(6)H(13)) and by powder X-ray crystallography (R = C(12)H(25)). A low-energy structure which can be written as Au(23)@(Au(SR)(2))(3)(Au(2)(SR)(3))(6) having a bi-icosahedral core and a chiral arrangement of the protecting gold-thiolate Au(x)(SR)(y) units yields an excellent match between the computed (for R = C(6)H(13)) and measured (for R = C(12)H(25)) powder X-ray diffraction function. We interpret in detail the electronic structure of the Au(23) core by using a particle-in-a-cylinder model. Although the alkane thiolate ligands are achiral, the chiral structure of the ligand layer yields strong circular dichroism (CD) in the excitations below 2.2 eV for Au(38)(SCH(3))(24). Our calculated CD spectrum is in quantitative agreement with the previously measured low-energy CD signal of glutathione-protected Au(38)(SG)(24). Our study demonstrates a new mechanism for the strong chiral response of thiolate-protected gold clusters with achiral metal cores and ligands.
中文翻译:

硫醇盐保护的Au38纳米团簇的手性和电子结构
通过密度泛函理论计算研究了硫醇盐保护的 Au(38)(SR)(24) 簇的结构、电子和光学特性(R = CH(3) 和 R = C(6)H(13))并通过粉末 X 射线晶体学 (R = C(12)H(25))。一种低能量结构,可以写成 Au(23)@(Au(SR)(2))(3)(Au(2)(SR)(3))(6) 具有双二十面体核和保护性金硫醇 Au(x)(SR)(y) 单元的手性排列在计算(对于 R = C(6)H(13))和测量(对于 R = C(12)H)之间产生了极好的匹配(25))粉末X射线衍射功能。我们通过使用圆柱体粒子模型详细解释了 Au(23) 核的电子结构。尽管烷烃硫醇盐配体是非手性的,但配体层的手性结构在低于 2.2 eV 的 Au(38)(SCH(3))(24) 激发中产生强圆二色性 (CD)。我们计算的 CD 光谱与先前测量的谷胱甘肽保护的 Au(38)(SG)(24) 的低能量 CD 信号定量一致。我们的研究证明了具有非手性金属核和配体的硫醇盐保护的金簇的强手性反应的新机制。
更新日期:2010-06-16
中文翻译:

硫醇盐保护的Au38纳米团簇的手性和电子结构
通过密度泛函理论计算研究了硫醇盐保护的 Au(38)(SR)(24) 簇的结构、电子和光学特性(R = CH(3) 和 R = C(6)H(13))并通过粉末 X 射线晶体学 (R = C(12)H(25))。一种低能量结构,可以写成 Au(23)@(Au(SR)(2))(3)(Au(2)(SR)(3))(6) 具有双二十面体核和保护性金硫醇 Au(x)(SR)(y) 单元的手性排列在计算(对于 R = C(6)H(13))和测量(对于 R = C(12)H)之间产生了极好的匹配(25))粉末X射线衍射功能。我们通过使用圆柱体粒子模型详细解释了 Au(23) 核的电子结构。尽管烷烃硫醇盐配体是非手性的,但配体层的手性结构在低于 2.2 eV 的 Au(38)(SCH(3))(24) 激发中产生强圆二色性 (CD)。我们计算的 CD 光谱与先前测量的谷胱甘肽保护的 Au(38)(SG)(24) 的低能量 CD 信号定量一致。我们的研究证明了具有非手性金属核和配体的硫醇盐保护的金簇的强手性反应的新机制。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号