当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
2,2′,4,4′-Tetrabromodiphenyl Ether (PBDE 47) Selectively Stimulates Proatherogenic PPARγ Signatures in Human THP-1 Macrophages to Contribute to Foam Cell Formation
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2022-05-16 , DOI: 10.1021/acs.chemrestox.2c00043 Qidong Ren 1, 2 , Xinni Xie 1 , Chuanfang Zhao 1 , Qing Wen 1, 2 , Ruiying Pan 1, 2 , Yuguo Du 1, 2
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2022-05-16 , DOI: 10.1021/acs.chemrestox.2c00043 Qidong Ren 1, 2 , Xinni Xie 1 , Chuanfang Zhao 1 , Qing Wen 1, 2 , Ruiying Pan 1, 2 , Yuguo Du 1, 2
Affiliation
|
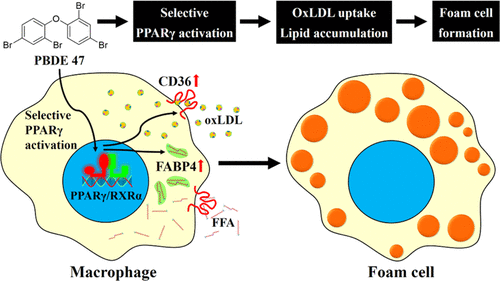
2,2′,4,4′-Tetrabromodiphenyl ether (PBDE 47) is one of the most prominent PBDE congeners detected in the human body, suggesting that the potential health risks of PBDE 47 should be thoroughly considered. However, the cardiovascular toxicity of PBDE 47 remains poorly understood. Here, toxic outcomes of PBDE 47 in human THP-1 macrophages concerning foam cell formation, which play crucial roles in the occurrence and development of atherosclerosis, were elucidated. First, our results indicated that PBDE 47 affected the PPARγ pathway most efficiently in THP-1 macrophages by transcriptomic analysis. Second, the PPARγ target genes CD36 and FABP4, responsible for lipid uptake and accumulation in macrophages, were consistently upregulated both at transcriptional and translational levels in THP-1 macrophages upon PBDE 47. Unexpectedly, PBDE 47 failed to activate the PPARγ target gene LXRα and PPARγ-LXRα-ABCA1/G1 cascade, which is activated by the PPARγ full agonist rosiglitazone and enables cholesterol efflux in macrophages. Thus, coincident with the selective upregulation of the PPARγ target genes CD36 and FABP4, PBDE 47, distinct from rosiglitazone, functionally resulted in more lipid accumulation and oxLDL uptake in THP-1 macrophages through high-content analysis (HCA). Moreover, these effects were markedly abrogated by the addition of the PPARγ antagonist T0070907. Mechanistically, the structural basis of selective activation of PPARγ by PBDE 47 was explored by molecular docking and dynamics simulation, which indicated that PBDE 47 interacted with the PPARγ ligand binding domain (PPARγ-LBD) distinctively from that of rosiglitazone. PBDE 47 was revealed to interact with helix 3 and helix 5 but not helix 12 in the PPARγ-LBD. Collectively, these results unraveled the potential cardiovascular toxicity of PBDE 47 by selective activation of PPARγ to facilitate foam cell formation for the first time.
中文翻译:

2,2',4,4'-四溴二苯醚 (PBDE 47) 选择性地刺激人类 THP-1 巨噬细胞中的致动脉粥样硬化 PPARγ 特征以促进泡沫细胞的形成
2,2',4,4'-四溴二苯醚 (PBDE 47) 是人体中检测到的最主要的 PBDE 同系物之一,这表明应该彻底考虑 PBDE 47 的潜在健康风险。然而,PBDE 47 的心血管毒性仍然知之甚少。在此,阐明了 PBDE 47 在人类 THP-1 巨噬细胞中与泡沫细胞形成有关的毒性结果,泡沫细胞在动脉粥样硬化的发生和发展中起关键作用。首先,我们的结果表明,通过转录组学分析,PBDE 47 在 THP-1 巨噬细胞中最有效地影响 PPARγ 途径。二、PPARγ靶基因CD36和FABP4,负责巨噬细胞中脂质的摄取和积累,在 PBDE 47 后,在 THP-1 巨噬细胞的转录和翻译水平上持续上调。出乎意料的是,PBDE 47 未能激活 PPARγ 靶基因LXRα和 PPARγ-LXRα-ABCA1/G1 级联反应,它由 PPARγ 完全激动剂罗格列酮激活,使巨噬细胞中的胆固醇流出。因此,与 PPARγ 靶基因CD36和FABP4的选择性上调一致, PBDE 47 与罗格列酮不同,通过高含量分析 (HCA) 在功能上导致 THP-1 巨噬细胞中更多的脂质积累和 oxLDL 摄取。此外,添加 PPARγ 拮抗剂 T0070907 后,这些效果明显消失。在机制上,通过分子对接和动力学模拟探索了 PBDE 47 选择性激活 PPARγ 的结构基础,这表明 PBDE 47 与 PPARγ 配体结合域 (PPARγ-LBD) 的相互作用不同于罗格列酮。显示 PBDE 47 与螺旋 3 和螺旋 5 相互作用,但不与 PPARγ-LBD 中的螺旋 12 相互作用。总的来说,这些结果首次揭示了 PBDE 47 通过选择性激活 PPARγ 以促进泡沫细胞形成的潜在心血管毒性。
更新日期:2022-05-16
中文翻译:

2,2',4,4'-四溴二苯醚 (PBDE 47) 选择性地刺激人类 THP-1 巨噬细胞中的致动脉粥样硬化 PPARγ 特征以促进泡沫细胞的形成
2,2',4,4'-四溴二苯醚 (PBDE 47) 是人体中检测到的最主要的 PBDE 同系物之一,这表明应该彻底考虑 PBDE 47 的潜在健康风险。然而,PBDE 47 的心血管毒性仍然知之甚少。在此,阐明了 PBDE 47 在人类 THP-1 巨噬细胞中与泡沫细胞形成有关的毒性结果,泡沫细胞在动脉粥样硬化的发生和发展中起关键作用。首先,我们的结果表明,通过转录组学分析,PBDE 47 在 THP-1 巨噬细胞中最有效地影响 PPARγ 途径。二、PPARγ靶基因CD36和FABP4,负责巨噬细胞中脂质的摄取和积累,在 PBDE 47 后,在 THP-1 巨噬细胞的转录和翻译水平上持续上调。出乎意料的是,PBDE 47 未能激活 PPARγ 靶基因LXRα和 PPARγ-LXRα-ABCA1/G1 级联反应,它由 PPARγ 完全激动剂罗格列酮激活,使巨噬细胞中的胆固醇流出。因此,与 PPARγ 靶基因CD36和FABP4的选择性上调一致, PBDE 47 与罗格列酮不同,通过高含量分析 (HCA) 在功能上导致 THP-1 巨噬细胞中更多的脂质积累和 oxLDL 摄取。此外,添加 PPARγ 拮抗剂 T0070907 后,这些效果明显消失。在机制上,通过分子对接和动力学模拟探索了 PBDE 47 选择性激活 PPARγ 的结构基础,这表明 PBDE 47 与 PPARγ 配体结合域 (PPARγ-LBD) 的相互作用不同于罗格列酮。显示 PBDE 47 与螺旋 3 和螺旋 5 相互作用,但不与 PPARγ-LBD 中的螺旋 12 相互作用。总的来说,这些结果首次揭示了 PBDE 47 通过选择性激活 PPARγ 以促进泡沫细胞形成的潜在心血管毒性。














































 京公网安备 11010802027423号
京公网安备 11010802027423号