Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2022-05-15 , DOI: 10.1016/j.apcatb.2022.121515
Yingyu Ren , Yusen Yang , Lifang Chen , Lei Wang , Yawen Shi , Pan Yin , Wenlong Wang , Mingfei Shao , Xin Zhang , Min Wei
|
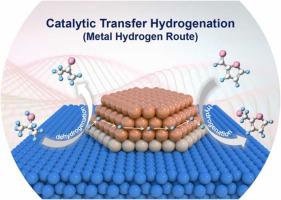
As a safe and environmentally friendly selective hydrogenation method, catalytic transfer hydrogenation (CTH) has aroused great interest in preparation of high-value-added products from biomass derived resources. Herein, a Cu-based catalyst (Cu/CuAl-MMO-400) was prepared by structural topological transformation of layered double hydroxides (LDHs) precursor, which displayed promising catalytic behavior toward CTH reaction of furfural (FAL) with 2-propanol (2-POL) as the hydrogen donor. Notably, the reaction rate is as high as 0.125 mol g−1 h−1, which is superior to previously reported non-noble metal catalysts. A combination investigation based on XPS, XANES and Bader charge confirms the co-existence of Cu0 and Cu+ sites on the surface of Cu nanoparticles. Both experimental studies (in situ DRIFTS and isotope labelling MS) and DFT calculations reveal that the Cu0−Cu+ synergistic effect plays a vital role in determining catalytic behavior: the Cu+ species acts as both dehydrogenation and hydrogenation active site; while the Cu0 site promotes the transfer of H atoms between adsorbed substrates. This work substantiates a Cu0 −Cu+ synergetic catalysis by establishing structure-property correlation and revealing reaction pathway, which could be extended to other CTH reactions in the upgrading processes of biomass.
中文翻译:

层状双氢氧化物衍生的Cu0-Cu+对催化转移加氢反应的协同作用
作为一种安全、环保的选择性加氢方法,催化转移加氢(CTH)在利用生物质衍生资源制备高附加值产品方面引起了人们的极大兴趣。本文通过层状双氢氧化物 (LDHs) 前驱体的结构拓扑转变制备了铜基催化剂 (Cu/CuAl-MMO-400),该催化剂对糠醛 (FAL) 与 2-丙醇 (2 -POL) 作为氢供体。值得注意的是,反应速率高达 0.125 mol g -1 h -1,优于先前报道的非贵金属催化剂。基于 XPS、XANES 和 Bader 电荷的组合研究证实了 Cu 0和 Cu +的共存Cu纳米粒子表面的位点。实验研究(原位DRIFTS 和同位素标记 MS)和 DFT 计算都表明,Cu 0 - Cu +协同效应在决定催化行为方面起着至关重要的作用:Cu +物质同时作为脱氢和加氢活性位点;而Cu 0位点促进了吸附基板之间的H原子转移。这项工作通过建立结构-性质相关性和揭示反应途径证实了Cu 0 -Cu +协同催化,这可以扩展到生物质升级过程中的其他CTH反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号