当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aqueous Electrolytes Reinforced by Mg and Ca Ions for Highly Reversible Fe Metal Batteries
ACS Central Science ( IF 12.7 ) Pub Date : 2022-05-12 , DOI: 10.1021/acscentsci.2c00293 Jing Liu 1 , Dengpan Dong 1 , Alan Larrea Caro 1 , Nicolai Sage Andreas 1 , Zongjian Li 1 , Yunan Qin 1 , Dimitry Bedrov 1 , Tao Gao 1
ACS Central Science ( IF 12.7 ) Pub Date : 2022-05-12 , DOI: 10.1021/acscentsci.2c00293 Jing Liu 1 , Dengpan Dong 1 , Alan Larrea Caro 1 , Nicolai Sage Andreas 1 , Zongjian Li 1 , Yunan Qin 1 , Dimitry Bedrov 1 , Tao Gao 1
Affiliation
|
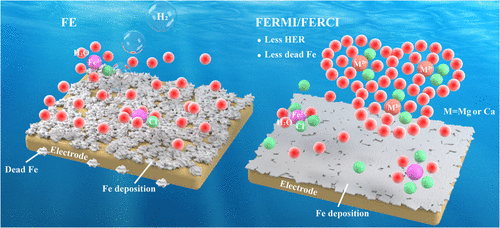
Iron (Fe) metal batteries, such as Fe-ion batteries and all Fe flow batteries, are promising energy storage technologies for grid applications due to the extremely low cost of Fe and Fe salts. Nonetheless, the cycle life of Fe metal batteries is poor primarily due to the low Coulombic efficiency of the Fe deposition/stripping reaction. Current aqueous electrolytes based on Fe chloride or sulfate salts can only operate at a Coulombic efficiency of <91% under mild operation conditions (<5 mA/cm2), largely due to undesired hydrogen evolution reaction (HER). This work reports a series of novel Fe electrolytes, Fe electrolytes reinforced with Mg ions (FERMI) and Ca ions (FERCI), which have remarkably better Coulombic efficiency, higher conductivity, and faster deposition/stripping kinetics. By the addition of 4.5 M MgCl2 or CaCl2 into the baseline FeCl2 electrolyte, the Fe deposition/stripping efficiency can be significantly improved to 99.1%, which greatly boosts the cycling performance of Fe metal batteries in both half-cells and full-cells. Mechanistic studies reveal that the remarkably improved efficiency is due to a reduced amount of “dead Fe” as well as suppressed HER. By the combination of experiments and molecular dynamics and density functional theory computation, the electrolyte structure is revealed, and the mechanism for enhanced water reduction resistance is elucidated. These novel electrolytes not only enable a highly reversible Fe metal anode for low-cost energy storage technologies but also have the potential to address the HER side reaction problem in other electrochemical technologies based on aqueous electrolytes, such as CO2 reduction, NH3 synthesis, etc.
中文翻译:

用于高可逆铁金属电池的 Mg 和 Ca 离子增强水系电解质
由于铁和铁盐的成本极低,铁(Fe)金属电池,如铁离子电池和全铁液流电池,是用于电网应用的有前途的储能技术。尽管如此,Fe金属电池的循环寿命很差,这主要是由于Fe沉积/剥离反应的库仑效率低。目前基于氯化铁或硫酸盐的水性电解质只能在温和的操作条件下(<5 mA/cm 2 )以 <91% 的库仑效率运行),主要是由于不希望的析氢反应 (HER)。这项工作报告了一系列新型 Fe 电解质,即用 Mg 离子 (FERMI) 和 Ca 离子 (FERCI) 增强的 Fe 电解质,它们具有显着更好的库仑效率、更高的电导率和更快的沉积/剥离动力学。通过将 4.5 M MgCl 2或 CaCl 2添加到基线 FeCl 2中在电解液中,Fe沉积/剥离效率可显着提高至99.1%,这极大地提高了Fe金属电池在半电池和全电池中的循环性能。机理研究表明,显着提高的效率是由于“死铁”数量的减少以及 HER 的抑制。通过实验与分子动力学和密度泛函理论计算相结合,揭示了电解质的结构,阐明了增强抗减水性的机理。这些新型电解质不仅能够为低成本储能技术提供高度可逆的 Fe 金属负极,而且还具有解决其他基于水性电解质的电化学技术中 HER 副反应问题的潜力,例如 CO 2还原、NH3合成等
更新日期:2022-05-12
中文翻译:

用于高可逆铁金属电池的 Mg 和 Ca 离子增强水系电解质
由于铁和铁盐的成本极低,铁(Fe)金属电池,如铁离子电池和全铁液流电池,是用于电网应用的有前途的储能技术。尽管如此,Fe金属电池的循环寿命很差,这主要是由于Fe沉积/剥离反应的库仑效率低。目前基于氯化铁或硫酸盐的水性电解质只能在温和的操作条件下(<5 mA/cm 2 )以 <91% 的库仑效率运行),主要是由于不希望的析氢反应 (HER)。这项工作报告了一系列新型 Fe 电解质,即用 Mg 离子 (FERMI) 和 Ca 离子 (FERCI) 增强的 Fe 电解质,它们具有显着更好的库仑效率、更高的电导率和更快的沉积/剥离动力学。通过将 4.5 M MgCl 2或 CaCl 2添加到基线 FeCl 2中在电解液中,Fe沉积/剥离效率可显着提高至99.1%,这极大地提高了Fe金属电池在半电池和全电池中的循环性能。机理研究表明,显着提高的效率是由于“死铁”数量的减少以及 HER 的抑制。通过实验与分子动力学和密度泛函理论计算相结合,揭示了电解质的结构,阐明了增强抗减水性的机理。这些新型电解质不仅能够为低成本储能技术提供高度可逆的 Fe 金属负极,而且还具有解决其他基于水性电解质的电化学技术中 HER 副反应问题的潜力,例如 CO 2还原、NH3合成等































 京公网安备 11010802027423号
京公网安备 11010802027423号