Molecular Therapy - Nucleic Acids ( IF 6.5 ) Pub Date : 2022-05-11 , DOI: 10.1016/j.omtn.2022.05.024 Katelyn A Doxtader Lacy 1 , Xue-Hai Liang 1 , Lingdi Zhang 1 , Stanley T Crooke 1
|
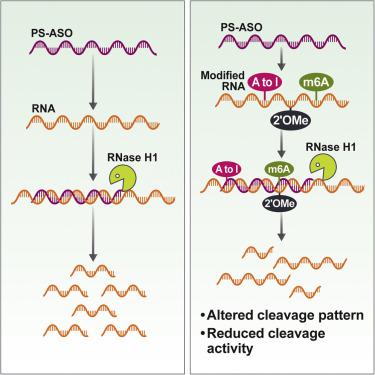
硫代磷酸酯修饰的反义寡核苷酸 (PS-ASO) 可以通过与靶 RNA 杂交并随后被 RNase H1 切割来降低基因表达。通过这种机制减少靶点受到 RNA 的许多特征的影响,这些特征调节 PS-ASO 与 RNA 靶点的结合亲和力,以及 PS-ASO-RNA 杂合体如何被 RNase H1 识别以进行 RNA 切割。内源性 RNA 经常经过化学修饰,可以调节 RNA 的分子内和分子间相互作用。PS-ASO 修饰对反义活性的影响已得到充分研究;然而,关于 RNA 修饰对 PS-ASO 杂交和 RNase H1 切割活性的影响知之甚少。这里,我们确定了三种不同 RNA 修饰对重组和基于细胞的系统中 PS-ASO 结合和反义活性的影响。一些 RNA 修饰可以降低 PS-ASO 杂交、RNase H1 的切割活性或两者兼而有之,而其他修饰对 PS-ASO 功能的影响最小。除了这些直接影响外,RNA 修饰还可以改变 RNA 结构,这可能会影响 PS-ASO 在细胞环境中的可及性。我们的研究结果阐明了三种流行的 RNA 修饰对 PS-ASO 介导的 RNase H1 切割活性的影响,这些发现将有助于改善 PS-ASO 靶位点选择。RNA 修饰也可以改变 RNA 结构,这可能会影响 PS-ASO 在细胞环境中的可及性。我们的研究结果阐明了三种流行的 RNA 修饰对 PS-ASO 介导的 RNase H1 切割活性的影响,这些发现将有助于改善 PS-ASO 靶位点选择。RNA 修饰也可以改变 RNA 结构,这可能会影响 PS-ASO 在细胞环境中的可及性。我们的研究结果阐明了三种流行的 RNA 修饰对 PS-ASO 介导的 RNase H1 切割活性的影响,这些发现将有助于改善 PS-ASO 靶位点选择。

"点击查看英文标题和摘要"































 京公网安备 11010802027423号
京公网安备 11010802027423号