Synthesis ( IF 2.2 ) Pub Date : 2022-03-22 , DOI: 10.1055/a-1804-8980 Stefan Herzog 1 , Inka Marten 1 , Aaron Weiß 1 , Joachim Podlech 1
|
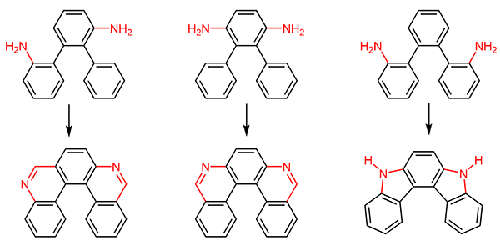
Double ortho-fusion in suitably substituted ortho-terphenyls was used for the synthesis of diaza[5]helicenes. Bis(carboxamido)-substituted ortho-terphenyls can be condensed to 5,9- and 6,9-diaza[5]helicenes, where substituents at the 6,10- and 5,10-positions, respectively, are introduced with the carboxamido groups. While a twofold coupling sequence with intermediate protection of one amino group has to be used for 5,9-diaza[5]helicenes, a more concise sequence avoiding the protection leads to 6,9-diaza[5]helicenes. The simple heating of ortho,ortho′-diazidoterphenyls furnishes 5,8-dihydroindolo[2,3-c]carbazoles, i.e., [5]helicenes with alternating benzene and pyrrole rings.
中文翻译:

邻三联苯的邻,邻'-融合合成二氮杂[5]螺烯
适当取代的邻三联苯中的双邻位融合用于合成二氮杂[5]螺烯。双(甲酰胺基)-取代的邻三联苯可以缩合为 5,9- 和 6,9-二氮杂 [5] 螺烯,其中 6,10- 和 5,10-位的取代基分别与甲酰胺基一起引入团体。虽然对于 5,9-二氮杂 [5] 螺旋烯必须使用具有一个氨基中间保护的双重偶联序列,但避免保护的更简洁的序列会导致 6,9-二氮杂 [5] 螺旋烯。邻位,邻位'-二叠氮三苯醚的简单加热提供了 5,8-二氢吲哚并[2,3- c ]咔唑,即具有交替苯环和吡咯环的 [5] 螺烯。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号