Chemosphere ( IF 8.1 ) Pub Date : 2022-05-10 , DOI: 10.1016/j.chemosphere.2022.134863 Ying Zhang 1 , Liming Zhang 1 , Jichang Lu 1 , Wei Liao 1 , Jin Zhang 2 , Xiaoya Gao 1 , Yongming Luo 3 , Wenjie Zhu 1
|
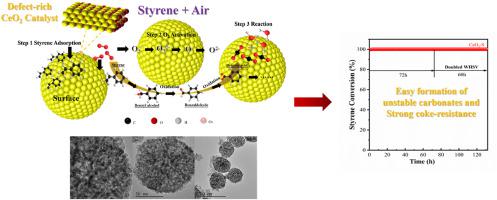
Spherical cerium dioxide (CeO2–S) nanoparticles were successfully prepared using a solvothermal method, and their performances in catalytic oxidation reactions were studied. The CeO2–S catalyst showed superior low-temperature catalytic activity for styrene removal (T90 = 118 °C, GHSV = 18,000 h−1) compared with commercial CeO2. The characterization results showed that there were numerous oxygen defects in CeO2–S that were key to its catalytic performance at low temperatures, high redox properties, and high adsorption capacity for the reaction gases (O2 and styrene). Moreover, the catalytic performance of CeO2–S was highly stable (132 h), and the particles were reusable. FTIR and in-situ DRIFTS results showed that the type of intermediates formed during the oxidation of styrene determined the CeO2 catalytic stability, and the main intermediates were bidentate carbonate species that accumulated on the surface of deactivated CeO2–S and were not thermally stable. Moreover, the soft carbon that also deposited on CeO2–S during the reaction was easily decomposed at higher temperatures. The role of the oxygen vacancies on the CeO2–S catalyst was further revealed by correlating the concentration of oxygen vacancies and the accumulation of coke on the catalyst surface.
中文翻译:

用于超低温催化氧化和持久脱除苯乙烯的富缺陷 CeO2 催化剂的研究
采用溶剂热法成功制备了球形二氧化铈(CeO 2 -S)纳米颗粒,并研究了它们在催化氧化反应中的性能。与商业CeO 2相比, CeO 2 -S 催化剂在去除苯乙烯方面表现出优异的低温催化活性(T 90 = 118 °C,GHSV = 18,000 h -1)。表征结果表明,CeO 2 -S 中存在许多氧缺陷,这是其在低温下的催化性能、高氧化还原性能和对反应气体(O 2和苯乙烯)具有高吸附能力的关键。此外,CeO 2的催化性能-S 高度稳定(132 小时),颗粒可重复使用。FTIR和原位DRIFTS结果表明,苯乙烯氧化过程中形成的中间体类型决定了CeO 2的催化稳定性,主要的中间体是在失活的CeO 2 -S表面积累的二齿碳酸盐物质,并且不是热稳定的。 . 此外,在反应过程中也沉积在 CeO 2 -S 上的软碳在较高温度下很容易分解。通过关联氧空位的浓度和催化剂表面焦炭的积累,进一步揭示了氧空位对 CeO 2 -S 催化剂的作用。















































 京公网安备 11010802027423号
京公网安备 11010802027423号