当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereocontrolled Synthesis of Fluorinated Isochromans via Iodine(I)/Iodine(III) Catalysis
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-05-10 , DOI: 10.1002/anie.202205277
Joel Häfliger 1 , Olga O Sokolova 1 , Madina Lenz 1 , Constantin G Daniliuc 1 , Ryan Gilmour 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-05-10 , DOI: 10.1002/anie.202205277
Joel Häfliger 1 , Olga O Sokolova 1 , Madina Lenz 1 , Constantin G Daniliuc 1 , Ryan Gilmour 1
Affiliation
|
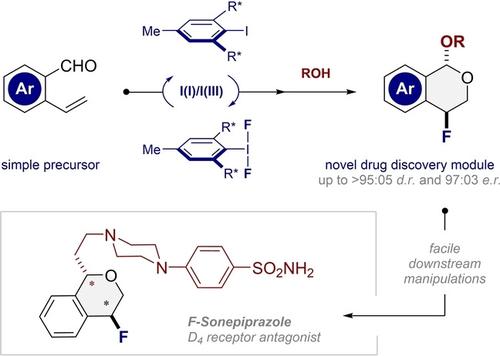
A chiral II/IIII catalysis platform is reported to facilitate the fluorocyclization of 2-vinyl benzaldehydes thereby generating novel fluorinated isochromans in a single operation (up to >95 : 05 d.r. and 97 : 03 e.r.). Not only does the benzylic fluoride shield an oxidatively labile position, X-ray analyses demonstrate that the [CH2-CHF] fragment functions as a stereoelectronic mimic of the O-CH(OR) acetal motif.
中文翻译:

碘(I)/碘(III)催化立体控制合成氟化异色满
据报道,手性 I/I III催化平台可促进 2-乙烯基苯甲醛的氟环化,从而在一次操作中生成新型氟化异色满(高达 >95: 05 dr . 和 97: 03 er .)。氟化苄不仅屏蔽了氧化不稳定的位置,X射线分析还表明[CH 2 -CHF]片段起到了O -CH(OR)乙缩醛基序的立体电子模拟物的作用。
更新日期:2022-05-10
中文翻译:

碘(I)/碘(III)催化立体控制合成氟化异色满
据报道,手性 I/I III催化平台可促进 2-乙烯基苯甲醛的氟环化,从而在一次操作中生成新型氟化异色满(高达 >95: 05 dr . 和 97: 03 er .)。氟化苄不仅屏蔽了氧化不稳定的位置,X射线分析还表明[CH 2 -CHF]片段起到了O -CH(OR)乙缩醛基序的立体电子模拟物的作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号