当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On-Resin Recognition of Aromatic Oligopeptides and Proteins through Host-Enhanced Heterodimerization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-05-10 , DOI: 10.1021/jacs.2c02287 Xiaoyi Chen 1 , Zehuan Huang 1 , Renata L Sala 1 , Alan M McLean 1 , Guanglu Wu 1 , Kamil Sokołowski 1 , Katie King 1 , Jade A McCune 1 , Oren A Scherman 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-05-10 , DOI: 10.1021/jacs.2c02287 Xiaoyi Chen 1 , Zehuan Huang 1 , Renata L Sala 1 , Alan M McLean 1 , Guanglu Wu 1 , Kamil Sokołowski 1 , Katie King 1 , Jade A McCune 1 , Oren A Scherman 1
Affiliation
|
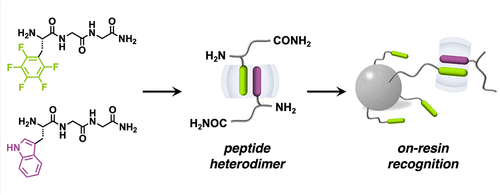
Peptide dimerization is ubiquitous in natural protein conjugates and artificial self-assemblies. A major challenge in artificial systems remains achieving quantitative peptide heterodimerization, critical for next-generation biomolecular purification and formulation of therapeutics. Here, we employ a synthetic host to simultaneously encapsulate an aromatic and a noncanonical l-perfluorophenylalanine-containing peptide through embedded polar−π interactions, constructing an unprecedented series of heteropeptide dimers. To demonstrate the utility, this heteropeptide dimerization strategy was applied toward on-resin recognition of N-terminal aromatic residues in peptides as well as insulin, both exhibiting high recycling efficiency (>95%). This research unveils a generic approach to exploit quantitative heteropeptide dimers for the design of supramolecular (bio)systems.
中文翻译:

通过宿主增强的异二聚化对芳香寡肽和蛋白质的树脂上识别
肽二聚化在天然蛋白质缀合物和人工自组装中普遍存在。人工系统的一个主要挑战仍然是实现定量肽异二聚化,这对于下一代生物分子纯化和治疗剂的制定至关重要。在这里,我们使用合成宿主通过嵌入的极性-π 相互作用同时封装芳香族和非经典的含l-全氟苯丙氨酸的肽,构建了前所未有的杂肽二聚体系列。为了证明实用性,这种异肽二聚化策略被应用于N的树脂上识别-肽和胰岛素中的末端芳香族残基,两者都表现出高回收效率(> 95%)。这项研究揭示了一种利用定量异肽二聚体设计超分子(生物)系统的通用方法。
更新日期:2022-05-10
中文翻译:

通过宿主增强的异二聚化对芳香寡肽和蛋白质的树脂上识别
肽二聚化在天然蛋白质缀合物和人工自组装中普遍存在。人工系统的一个主要挑战仍然是实现定量肽异二聚化,这对于下一代生物分子纯化和治疗剂的制定至关重要。在这里,我们使用合成宿主通过嵌入的极性-π 相互作用同时封装芳香族和非经典的含l-全氟苯丙氨酸的肽,构建了前所未有的杂肽二聚体系列。为了证明实用性,这种异肽二聚化策略被应用于N的树脂上识别-肽和胰岛素中的末端芳香族残基,两者都表现出高回收效率(> 95%)。这项研究揭示了一种利用定量异肽二聚体设计超分子(生物)系统的通用方法。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号