当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Volatile Products from Ligand Addition of P(CH3)3 to NiCl2, PdCl2, and PtCl2: Pathway for Metal Thermal Atomic Layer Etching
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-05-09 , DOI: 10.1021/acs.jpcc.1c10690
Ann Lii-Rosales 1 , Virginia L. Johnson 1 , Sandeep Sharma 1 , Andreas Fischer 2 , Thorsten Lill 2 , Steven M. George 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-05-09 , DOI: 10.1021/acs.jpcc.1c10690
Ann Lii-Rosales 1 , Virginia L. Johnson 1 , Sandeep Sharma 1 , Andreas Fischer 2 , Thorsten Lill 2 , Steven M. George 1
Affiliation
|
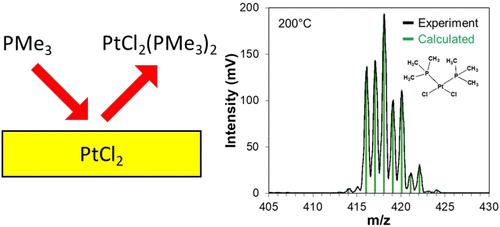
NiCl2, PdCl2, and PtCl2 can be spontaneously etched by ligand addition with P(CH3)3 (P(Me)3, trimethylphosphine). Ligand addition of P(Me)3 to these group 10 metal chlorides can form stable and volatile complexes. These ligand-addition reactions are suggested by the covalent bond classification (CBC) method. The CBC method provides guidelines for determining probable transition metal complexes based upon the stoichiometry of the metal (M), one-electron donor ligands (X), and electron-pair donor ligands (L). The CBC model predicts that a complex with a stoichiometry of MX2L2 is most frequently observed for Ni, Pd, and Pt. This prediction suggests that MCl2(P(Me)3)2 is a likely stable product after exposing Ni, Pd, and Pt to chlorination and then ligand-addition reactions with P(Me)3. In accordance with these expectations, in situ QMS studies revealed that NiCl2(P(Me)3)2+, PdCl2(P(Me)3)2+, and PtCl2(P(Me)3)2+ were observed as volatile metal complexes resulting from P(Me)3 exposure to NiCl2, PdCl2, and PtCl2. Thermochemical calculations also indicated that addition of P(Me)3 to MCl2 and MCl2(P(Me)3) was favorable where M = Ni, Pd, and Pt. The identification of these volatile products and the theoretical verification of their stability suggest that pathways for Ni, Pd, and Pt thermal atomic layer etching (ALE) are possible based on sequential chlorination and ligand-exchange reactions.
中文翻译:

P(CH3)3 配体添加到 NiCl2、PdCl2 和 PtCl2 中的挥发性产物:金属热原子层蚀刻的途径
NiCl 2、PdCl 2和PtCl 2可以通过与P(CH 3 ) 3 (P(Me) 3,三甲基膦)的配体添加而自发蚀刻。P(Me) 3与这些第 10 族金属氯化物的配体加成可形成稳定且易挥发的配合物。这些配体加成反应由共价键分类 (CBC) 方法提出。CBC 方法为根据金属 (M)、单电子供体配体 (X) 和电子对供体配体 (L) 的化学计量确定可能的过渡金属配合物提供了指南。CBC 模型预测具有 MX 2 L 2化学计量的复合物Ni、Pd 和 Pt 最常见。这一预测表明,在将 Ni、Pd 和 Pt 暴露于氯化,然后与 P(Me) 3 发生配体加成反应后, MCl 2 (P(Me) 3 ) 2可能是一种稳定的产物。根据这些预期,原位QMS 研究表明,观察到 NiCl 2 ( P(Me) 3 ) 2 +、PdCl 2 (P(Me) 3 ) 2 +和 PtCl 2 (P(Me) 3 ) 2 +作为由 P(Me) 3产生的挥发性金属配合物暴露于 NiCl 2、 PdCl 2和 PtCl 2。热化学计算还表明,将 P(Me) 3添加到 MCl 2和 MCl 2 (P(Me) 3 ) 是有利的,其中 M = Ni、Pd 和 Pt。这些挥发性产物的鉴定及其稳定性的理论验证表明,基于连续氯化和配体交换反应的 Ni、Pd 和 Pt 热原子层蚀刻 (ALE) 途径是可能的。
更新日期:2022-05-09
中文翻译:

P(CH3)3 配体添加到 NiCl2、PdCl2 和 PtCl2 中的挥发性产物:金属热原子层蚀刻的途径
NiCl 2、PdCl 2和PtCl 2可以通过与P(CH 3 ) 3 (P(Me) 3,三甲基膦)的配体添加而自发蚀刻。P(Me) 3与这些第 10 族金属氯化物的配体加成可形成稳定且易挥发的配合物。这些配体加成反应由共价键分类 (CBC) 方法提出。CBC 方法为根据金属 (M)、单电子供体配体 (X) 和电子对供体配体 (L) 的化学计量确定可能的过渡金属配合物提供了指南。CBC 模型预测具有 MX 2 L 2化学计量的复合物Ni、Pd 和 Pt 最常见。这一预测表明,在将 Ni、Pd 和 Pt 暴露于氯化,然后与 P(Me) 3 发生配体加成反应后, MCl 2 (P(Me) 3 ) 2可能是一种稳定的产物。根据这些预期,原位QMS 研究表明,观察到 NiCl 2 ( P(Me) 3 ) 2 +、PdCl 2 (P(Me) 3 ) 2 +和 PtCl 2 (P(Me) 3 ) 2 +作为由 P(Me) 3产生的挥发性金属配合物暴露于 NiCl 2、 PdCl 2和 PtCl 2。热化学计算还表明,将 P(Me) 3添加到 MCl 2和 MCl 2 (P(Me) 3 ) 是有利的,其中 M = Ni、Pd 和 Pt。这些挥发性产物的鉴定及其稳定性的理论验证表明,基于连续氯化和配体交换反应的 Ni、Pd 和 Pt 热原子层蚀刻 (ALE) 途径是可能的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号