当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversed-Polarity Synthesis of N-Sulfonyl Ketimines with Imidoylsilanes and Diaryliodonium Salts via Palladium-Catalyzed Reactions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-05-09 , DOI: 10.1021/acs.joc.2c00116 Seungmi Lee 1 , Inji Shin 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-05-09 , DOI: 10.1021/acs.joc.2c00116 Seungmi Lee 1 , Inji Shin 1
Affiliation
|
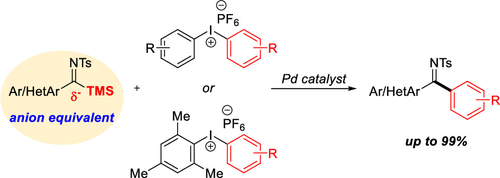
Imines are important building blocks in organic chemistry. Iminines, aldimines and ketimines, have been traditionally synthesized by the condensation reaction of the corresponding carbonyl compound with an amine moiety. More recently, palladium-catalyzed synthesis of ketimines using imidoyl chlorides has been reported. As an alternative, we report the reversed-polarity synthesis of N-sulfonyl ketimines using an anion equivalent imidoylsilane as a new nucleophilic coupling partner in the presence of a palladium catalyst. To the best of our knowledge, this is the first report of the use of imidoylsilanes in transition-metal-catalyzed coupling reactions. Diaryliodonium salt as an electrophile, various aryl–aryl or heteroaryl–aryl N-sulfonyl ketimines were successfully prepared in up to 99% isolated yields.
中文翻译:

钯催化反应反极性合成 N-磺酰基酮亚胺与亚氨基硅烷和二芳基碘鎓盐
亚胺是有机化学中的重要组成部分。亚胺、醛亚胺和酮亚胺传统上是通过相应的羰基化合物与胺部分的缩合反应合成的。最近,已经报道了使用亚胺酰氯的钯催化合成酮亚胺。作为替代方案,我们报告了在钯催化剂存在下使用阴离子等效的亚氨基硅烷作为新的亲核偶联配偶体的N-磺酰基酮亚胺的反极性合成。据我们所知,这是首次报道在过渡金属催化的偶联反应中使用亚氨基硅烷。二芳基碘盐作为亲电试剂,各种芳基-芳基或杂芳基-芳基N以高达 99% 的分离产率成功制备了 -磺酰酮亚胺。
更新日期:2022-05-09
中文翻译:

钯催化反应反极性合成 N-磺酰基酮亚胺与亚氨基硅烷和二芳基碘鎓盐
亚胺是有机化学中的重要组成部分。亚胺、醛亚胺和酮亚胺传统上是通过相应的羰基化合物与胺部分的缩合反应合成的。最近,已经报道了使用亚胺酰氯的钯催化合成酮亚胺。作为替代方案,我们报告了在钯催化剂存在下使用阴离子等效的亚氨基硅烷作为新的亲核偶联配偶体的N-磺酰基酮亚胺的反极性合成。据我们所知,这是首次报道在过渡金属催化的偶联反应中使用亚氨基硅烷。二芳基碘盐作为亲电试剂,各种芳基-芳基或杂芳基-芳基N以高达 99% 的分离产率成功制备了 -磺酰酮亚胺。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号