当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalysis with Diboron(4)/Pyridine: Application to the Broad-Scope [3 + 2] Cycloaddition of Cyclopropanes and Alkenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-05-09 , DOI: 10.1021/jacs.2c03673 Zhengwei Ding 1 , Zhi Liu 2 , Zhijun Wang 3 , Tao Yu 2 , Ming Xu 2 , Jingru Wen 2 , Kaiyan Yang 2 , Hailong Zhang 1 , Liang Xu 3 , Pengfei Li 2, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-05-09 , DOI: 10.1021/jacs.2c03673 Zhengwei Ding 1 , Zhi Liu 2 , Zhijun Wang 3 , Tao Yu 2 , Ming Xu 2 , Jingru Wen 2 , Kaiyan Yang 2 , Hailong Zhang 1 , Liang Xu 3 , Pengfei Li 2, 4
Affiliation
|
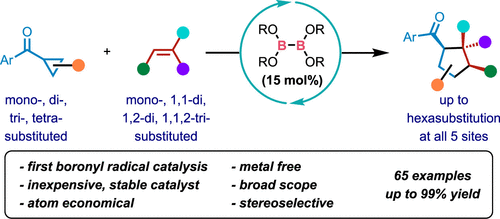
In contrast to the extensive but non-recyclable use of tetraalkoxydiboron(4) compounds as stoichiometric reagents in diverse reactions, this article reports an atom-economical reaction using a commercial diboron(4) as the catalyst. The key to success was designing a catalytic cycle for radical [3 + 2] cycloaddition involving a pyridine cocatalyst to generate from the diboron(4) catalyst and reversibly mediate the transfer of boronyl radicals. In comparison with known [3 + 2] cycloaddition with transition metal-based catalysts, the current reaction features not only metal-free conditions, inexpensive and stable catalysts, and simple operation but also remarkably broadened substrate scope. In particular, previously unusable cyclopropyl ketones without an activating group and/or alkenes with 1,2-disubstitution and 1,1,2-trisubstitution patterns were successfully used for the first time. Consequently, challenging cyclopentane compounds with various levels of substitution (65 examples, 57 new products, up to six substituents at all five ring atoms) were readily prepared in generally high to excellent yield and diastereoselectivity. The reaction was also successfully applied in concise formal synthesis of an anti-obesity drug and building natural product-like complex bridged or spirocyclic compounds. Mechanistic experiments and computational investigation support the proposed radical relay catalysis featuring a pyridine-assisted boronyl radical catalyst. Overall, this work demonstrates the first approach to use tetraalkoxydiboron(4) compounds as catalysts and may lead to the development of new, green, and efficient transition metal-like boron-catalyzed organic reactions.
中文翻译:

Diboron(4)/吡啶催化:在环丙烷和烯烃的广泛 [3 + 2] 环加成中的应用
与广泛但不可回收地使用四烷氧基二硼 (4) 化合物作为化学计量试剂在各种反应中相比,本文报告了使用商业二硼 (4) 作为催化剂的原子经济反应。成功的关键是为自由基 [3 + 2] 环加成设计一个催化循环,其中涉及吡啶助催化剂从二硼 (4) 催化剂生成并可逆地介导硼基自由基的转移。与已知的[3+2]过渡金属基催化剂的环加成反应相比,目前的反应不仅具有无金属条件、催化剂廉价稳定、操作简单等特点,而且显着拓宽了底物范围。特别是,以前无法使用的没有活化基团的环丙基酮和/或具有 1,2-二取代和 1,1 的烯烃,2-三取代模式首次成功使用。因此,具有各种取代水平的具有挑战性的环戊烷化合物(65 个实例,57 个新产品,在所有五个环原子上最多有六个取代基)很容易以通常高至优异的产率和非对映选择性制备。该反应还成功地应用于抗肥胖药物的简明形式合成和构建类似天然产物的复杂桥接或螺环化合物。机械实验和计算研究支持所提出的自由基中继催化,其特征在于吡啶辅助硼基自由基催化剂。总体而言,这项工作展示了使用四烷氧基二硼(4)化合物作为催化剂的第一种方法,并可能导致开发新的、绿色的、
更新日期:2022-05-09
中文翻译:

Diboron(4)/吡啶催化:在环丙烷和烯烃的广泛 [3 + 2] 环加成中的应用
与广泛但不可回收地使用四烷氧基二硼 (4) 化合物作为化学计量试剂在各种反应中相比,本文报告了使用商业二硼 (4) 作为催化剂的原子经济反应。成功的关键是为自由基 [3 + 2] 环加成设计一个催化循环,其中涉及吡啶助催化剂从二硼 (4) 催化剂生成并可逆地介导硼基自由基的转移。与已知的[3+2]过渡金属基催化剂的环加成反应相比,目前的反应不仅具有无金属条件、催化剂廉价稳定、操作简单等特点,而且显着拓宽了底物范围。特别是,以前无法使用的没有活化基团的环丙基酮和/或具有 1,2-二取代和 1,1 的烯烃,2-三取代模式首次成功使用。因此,具有各种取代水平的具有挑战性的环戊烷化合物(65 个实例,57 个新产品,在所有五个环原子上最多有六个取代基)很容易以通常高至优异的产率和非对映选择性制备。该反应还成功地应用于抗肥胖药物的简明形式合成和构建类似天然产物的复杂桥接或螺环化合物。机械实验和计算研究支持所提出的自由基中继催化,其特征在于吡啶辅助硼基自由基催化剂。总体而言,这项工作展示了使用四烷氧基二硼(4)化合物作为催化剂的第一种方法,并可能导致开发新的、绿色的、















































 京公网安备 11010802027423号
京公网安备 11010802027423号