当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Weak Brønsted base-promoted photoredox catalysis for CH alkylation of heteroarenes mediated by triplet excited diaryl ketone
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2022-05-07 , DOI: 10.1016/j.tetlet.2022.153846 Lifan Li 1 , Xuyan Song 2 , Mei-Fang Qi 1 , Bing Sun 1
中文翻译:

弱布朗斯台德碱促进光氧化还原催化三重激发二芳基酮介导的杂芳烃 CH 烷基化
更新日期:2022-05-07
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2022-05-07 , DOI: 10.1016/j.tetlet.2022.153846 Lifan Li 1 , Xuyan Song 2 , Mei-Fang Qi 1 , Bing Sun 1
Affiliation
|
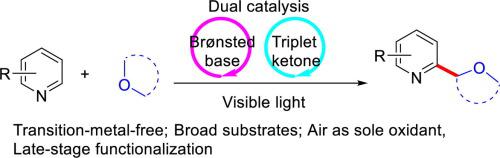
A weak Brønsted base-promoted photoredox catalysis has been developed for the direct C H α-alkylation of heteroarenes with cyclic and acyclic ethers. The high efficiency of this strategy is demonstrated by the mild reaction conditions, broad substrate scope, economical reagents and high regioselectivity. With air as the sole oxidant, a set of alkylated heteroarenes were accessed smoothly. This strategy was also applied for late-stage functionalization of valuable vitamin E nicotinate and loratadine.
H α-alkylation of heteroarenes with cyclic and acyclic ethers. The high efficiency of this strategy is demonstrated by the mild reaction conditions, broad substrate scope, economical reagents and high regioselectivity. With air as the sole oxidant, a set of alkylated heteroarenes were accessed smoothly. This strategy was also applied for late-stage functionalization of valuable vitamin E nicotinate and loratadine.
中文翻译:

弱布朗斯台德碱促进光氧化还原催化三重激发二芳基酮介导的杂芳烃 CH 烷基化
已经开发了一种弱 Brønsted 碱促进的光氧化还原催化,用于 杂芳烃与环状和无环醚的直接 C H α-烷基化。该策略的高效性体现在温和的反应条件、广泛的底物范围、经济的试剂和高区域选择性。以空气为唯一氧化剂,顺利获得了一组烷基化杂芳烃。该策略也适用于有价值的维生素 E 烟酸酯和氯雷他定的后期功能化。
杂芳烃与环状和无环醚的直接 C H α-烷基化。该策略的高效性体现在温和的反应条件、广泛的底物范围、经济的试剂和高区域选择性。以空气为唯一氧化剂,顺利获得了一组烷基化杂芳烃。该策略也适用于有价值的维生素 E 烟酸酯和氯雷他定的后期功能化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号