Process Biochemistry ( IF 3.7 ) Pub Date : 2022-05-06 , DOI: 10.1016/j.procbio.2022.05.004 Xiaojie Liu 1, 2 , Yifan Hu 2 , Bin Wei 1, 2 , Fang Liu 1, 2 , Haichang Xu 1, 2 , Changxia Liu 2 , Ye Li 3 , Hao Liang 1, 2
|
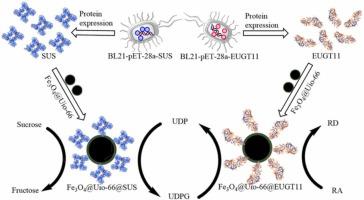
Rebaudioside D (RD) is a natural low-calorie, high-sweet sweetener and an excellent sugar substitute. In this study, RD was produced by coupling glucosyltransferase (EUGT11) and sucrose synthase (SUS) with a novel strategy of immobilization to enhance the recyclability of enzymes and decrease the production costs of RD. Recombinant EUGT11 from Oryza sativa and SUS from Arabidopsis thaliana were expressed in Escherichia coli and immobilized onto Fe3O4@Uio-66 nanocomposite. The results of Fourier transform Infrared spectra (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), magnetic susceptibility (VSM), thermogravimetric (TG) analysis and Brunauer–Emmett–Teller (BET) indicated that the magnetic nanocomposite Fe3O4@Uio-66 was successfully fabricated and the two enzymes were separately immobilized on Fe3O4@Uio-66. The reusability, storage, pH and temperature stabilities of the immobilized enzymes were investigated and compared to that of free enzymes. It was more stable towards temperature compared with free enzyme. The kinetic properties of immobilized EUGT11 showed a lower Vm and a higher Km compared to free EUGT11, and immobilized SUS showed a lower Vm and Km compared to free SUS. The immobilized SUS had around 56% residual activity upon storage a period of 10 days at 4 °C. After 8 times, the catalytic activity of immobilized double enzyme still retained around 80%, which showed a desirable stability and reduced the overall cost of enzymes, indicating that the immobilized enzymes had a good industrial application prospect in RD production.
中文翻译:

固定化葡萄糖基转移酶和蔗糖合酶在 Fe3O4@Uio-66 上的级联催化用于从莱鲍迪苷 A 一锅转化莱鲍迪苷 D
莱鲍迪甙 D (RD) 是一种天然的低热量、高甜味甜味剂,是一种极好的糖替代品。在这项研究中,RD 是通过将葡糖基转移酶 (EUGT11) 和蔗糖合酶 (SUS) 与一种新的固定化策略偶联产生的,以提高酶的可回收性并降低 RD 的生产成本。来自Oryza sativa的重组 EUGT11和来自拟南芥的SUS在大肠杆菌中表达并固定在 Fe 3 O 4上@Uio-66 纳米复合材料。傅里叶变换红外光谱 (FT-IR)、X 射线衍射 (XRD)、扫描电子显微镜 (SEM)、透射电子显微镜 (TEM)、磁化率 (VSM)、热重 (TG) 分析和 Brunauer-Emmett 的结果–Teller (BET) 指出磁性纳米复合材料 Fe 3 O 4 @Uio-66 已成功制造,两种酶分别固定在 Fe 3 O 4 @Uio-66 上。研究了固定化酶的可重复使用性、储存、pH 和温度稳定性,并与游离酶进行了比较。与游离酶相比,它对温度更稳定。固定化 EUGT11 的动力学特性显示出较低的 V m和较高的 Km与游离 EUGT11 相比,固定化 SUS 与游离 SUS 相比显示出较低的 V m和 K m。固定化的 SUS 在 4°C 下储存 10 天后具有约 56% 的残留活性。8次后,固定化双酶的催化活性仍保持在80%左右,表现出较好的稳定性,降低了酶的综合成本,表明固定化酶在RD生产中具有良好的工业应用前景。

































 京公网安备 11010802027423号
京公网安备 11010802027423号