当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Production of afucosylated antibodies in CHO cells by coexpression of an anti-FUT8 intrabody
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2022-05-04 , DOI: 10.1002/bit.28127
Simon Joubert 1 , Julie Guimond 1 , Sylvie Perret 1 , Félix Malenfant 1 , S Mehdy Elahi 1 , Anne Marcil 1 , Marie Parat 1 , Michel Gilbert 2 , Anne E G Lenferink 1 , Jason Baardsnes 1 , Yves Durocher 1
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2022-05-04 , DOI: 10.1002/bit.28127
Simon Joubert 1 , Julie Guimond 1 , Sylvie Perret 1 , Félix Malenfant 1 , S Mehdy Elahi 1 , Anne Marcil 1 , Marie Parat 1 , Michel Gilbert 2 , Anne E G Lenferink 1 , Jason Baardsnes 1 , Yves Durocher 1
Affiliation
|
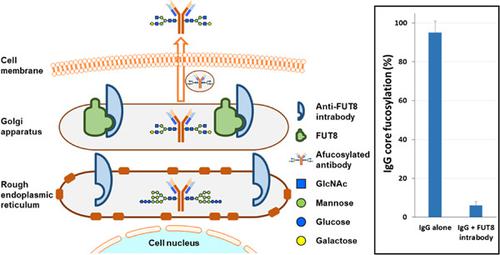
Some effector functions prompted by immunoglobulin G (IgG) antibodies, such as antibody-dependent cell-mediated cytotoxicity (ADCC), strongly depend on the N-glycans linked to asparagine 297 of the Fc region of the protein. A single α-(1,6)-fucosyltransferase (FUT8) is responsible for catalyzing the addition of an α-1,6-linked fucose residue to the first GlcNAc residue of the N-linked glycans. Antibodies missing this core fucose show a significantly enhanced ADCC and increased antitumor activity, which could help reduce therapeutic dose requirement, potentially translating into reduced safety concerns and manufacturing costs. Several approaches have been developed to modify glycans and improve the biological functions of antibodies. Here, we demonstrate that expression of a membrane-associated anti-FUT8 intrabody engineered to reside in the endoplasmic reticulum and Golgi apparatus can efficiently reduce FUT8 activity and therefore the core-fucosylation of the Fc N-glycan of an antibody. IgG1-producing CHO cells expressing the intrabody secrete antibodies with reduced core fucosylation as demonstrated by lectin blot analysis and UPLC-HILIC glycan analysis. Cells engineered to inhibit directly and specifically alpha-(1,6)-fucosyltransferase activity allows for the production of g/L levels of IgGs with strongly enhanced ADCC effector function, for which the level of fucosylation can be selected. The quick and efficient method described here should have broad practical applicability for the development of next-generation therapeutic antibodies with enhanced effector functions.
中文翻译:

通过抗 FUT8 内体共表达在 CHO 细胞中产生无岩藻糖基化抗体
由免疫球蛋白 G (IgG) 抗体引发的一些效应器功能,例如抗体依赖性细胞介导的细胞毒性 (ADCC),强烈依赖于与蛋白质 Fc 区天冬酰胺 297 相连的 N-聚糖。单个 α-(1,6)-岩藻糖基转移酶 (FUT8) 负责催化将 α-1,6-连接的岩藻糖残基添加到 N-连接聚糖的第一个 GlcNAc 残基上。缺少这种核心岩藻糖的抗体显示出显着增强的 ADCC 和增强的抗肿瘤活性,这可能有助于减少治疗剂量需求,从而可能转化为降低安全问题和制造成本。已经开发了几种方法来修饰聚糖和改善抗体的生物学功能。这里,我们证明了设计为驻留在内质网和高尔基体中的膜相关抗 FUT8 内体的表达可以有效地降低 FUT8 活性,从而降低抗体 Fc N-聚糖的核心岩藻糖基化。凝集素印迹分析和 UPLC-HILIC 聚糖分析表明,表达抗体的产生 IgG1 的 CHO 细胞分泌核心岩藻糖基化降低的抗体。设计为直接且特异性地抑制 α-(1,6)-岩藻糖基转移酶活性的细胞允许产生 g/L 水平的 IgG,具有强烈增强的 ADCC 效应功能,可以选择岩藻糖基化水平。这里描述的快速有效的方法对开发具有增强效应功能的下一代治疗性抗体具有广泛的实际适用性。
更新日期:2022-05-04
中文翻译:

通过抗 FUT8 内体共表达在 CHO 细胞中产生无岩藻糖基化抗体
由免疫球蛋白 G (IgG) 抗体引发的一些效应器功能,例如抗体依赖性细胞介导的细胞毒性 (ADCC),强烈依赖于与蛋白质 Fc 区天冬酰胺 297 相连的 N-聚糖。单个 α-(1,6)-岩藻糖基转移酶 (FUT8) 负责催化将 α-1,6-连接的岩藻糖残基添加到 N-连接聚糖的第一个 GlcNAc 残基上。缺少这种核心岩藻糖的抗体显示出显着增强的 ADCC 和增强的抗肿瘤活性,这可能有助于减少治疗剂量需求,从而可能转化为降低安全问题和制造成本。已经开发了几种方法来修饰聚糖和改善抗体的生物学功能。这里,我们证明了设计为驻留在内质网和高尔基体中的膜相关抗 FUT8 内体的表达可以有效地降低 FUT8 活性,从而降低抗体 Fc N-聚糖的核心岩藻糖基化。凝集素印迹分析和 UPLC-HILIC 聚糖分析表明,表达抗体的产生 IgG1 的 CHO 细胞分泌核心岩藻糖基化降低的抗体。设计为直接且特异性地抑制 α-(1,6)-岩藻糖基转移酶活性的细胞允许产生 g/L 水平的 IgG,具有强烈增强的 ADCC 效应功能,可以选择岩藻糖基化水平。这里描述的快速有效的方法对开发具有增强效应功能的下一代治疗性抗体具有广泛的实际适用性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号