当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic N-Allylation of Primary and Secondary Amines Using Renewable Cinnamic Acids Enabled by Bacterial Reductive Aminases
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-05-06 , DOI: 10.1021/acssuschemeng.2c01180
Godwin A Aleku 1, 2 , Gabriel R Titchiner 1 , George W Roberts 1 , Sasha R Derrington 1 , James R Marshall 1 , Florian Hollfelder 2 , Nicholas J Turner 1 , David Leys 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-05-06 , DOI: 10.1021/acssuschemeng.2c01180
Godwin A Aleku 1, 2 , Gabriel R Titchiner 1 , George W Roberts 1 , Sasha R Derrington 1 , James R Marshall 1 , Florian Hollfelder 2 , Nicholas J Turner 1 , David Leys 1
Affiliation
|
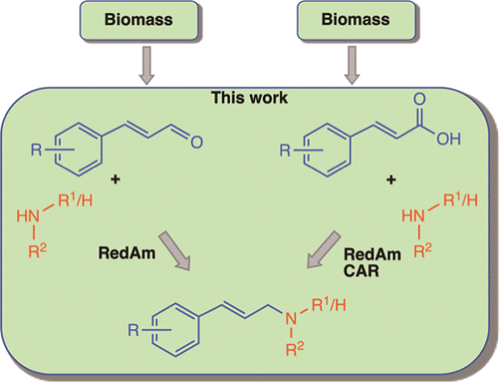
Allylic amines are a versatile class of synthetic precursors of many valuable nitrogen-containing organic compounds, including pharmaceuticals. Enzymatic allylic amination methods provide a sustainable route to these compounds but are often restricted to allylic primary amines. We report a biocatalytic system for the reductive N-allylation of primary and secondary amines, using biomass-derivable cinnamic acids. The two-step one-pot system comprises an initial carboxylate reduction step catalyzed by a carboxylic acid reductase to generate the corresponding α,β-unsaturated aldehyde in situ. This is followed by reductive amination of the aldehyde catalyzed by a bacterial reductive aminase pIR23 or BacRedAm to yield the corresponding allylic amine. We exploited pIR23, a prototype bacterial reductive aminase, self-sufficient in catalyzing formal reductive amination of α,β-unsaturated aldehydes with various amines, generating a broad range of secondary and tertiary amines accessed in up to 94% conversion under mild reaction conditions. Analysis of products isolated from preparative reactions demonstrated that only selective hydrogenation of the C═N bond had occurred, preserving the adjacent alkene moiety. This process represents an environmentally benign and sustainable approach for the synthesis of secondary and tertiary allylic amine frameworks, using renewable allylating reagents and avoiding harsh reaction conditions. The selectivity of the system ensures that bis-allylation of the alkylamines and (over)reduction of the alkene moiety are avoided.
中文翻译:

使用细菌还原氨基酶实现的可再生肉桂酸对伯胺和仲胺进行酶促 N-烯丙基化
烯丙胺是许多有价值的含氮有机化合物(包括药物)的一类多功能合成前体。酶促烯丙基胺化方法为这些化合物提供了可持续的途径,但通常仅限于烯丙基伯胺。我们报告了一种使用生物质衍生的肉桂酸对伯胺和仲胺进行还原性N-烯丙基化的生物催化系统。两步一锅系统包括由羧酸还原酶催化的初始羧酸还原步骤,以原位生成相应的α,β-不饱和醛。随后由细菌还原氨基酶 pIR23 或 BacRedAm 催化醛的还原胺化,产生相应的烯丙胺。我们利用 pIR23,一种原型细菌还原胺酶,能够自给自足地催化 α,β-不饱和醛与各种胺的正式还原胺化,生成多种仲胺和叔胺,在温和的反应条件下转化率高达 94%。对制备反应中分离的产物的分析表明,仅发生了 C=N 键的选择性氢化,保留了相邻的烯烃部分。该工艺代表了一种环境友好且可持续的合成仲和叔烯丙胺骨架的方法,使用可再生的烯丙基化试剂并避免苛刻的反应条件。该系统的选择性确保避免烷基胺的双烯丙基化和烯烃部分的(过度)还原。
更新日期:2022-05-06
中文翻译:

使用细菌还原氨基酶实现的可再生肉桂酸对伯胺和仲胺进行酶促 N-烯丙基化
烯丙胺是许多有价值的含氮有机化合物(包括药物)的一类多功能合成前体。酶促烯丙基胺化方法为这些化合物提供了可持续的途径,但通常仅限于烯丙基伯胺。我们报告了一种使用生物质衍生的肉桂酸对伯胺和仲胺进行还原性N-烯丙基化的生物催化系统。两步一锅系统包括由羧酸还原酶催化的初始羧酸还原步骤,以原位生成相应的α,β-不饱和醛。随后由细菌还原氨基酶 pIR23 或 BacRedAm 催化醛的还原胺化,产生相应的烯丙胺。我们利用 pIR23,一种原型细菌还原胺酶,能够自给自足地催化 α,β-不饱和醛与各种胺的正式还原胺化,生成多种仲胺和叔胺,在温和的反应条件下转化率高达 94%。对制备反应中分离的产物的分析表明,仅发生了 C=N 键的选择性氢化,保留了相邻的烯烃部分。该工艺代表了一种环境友好且可持续的合成仲和叔烯丙胺骨架的方法,使用可再生的烯丙基化试剂并避免苛刻的反应条件。该系统的选择性确保避免烷基胺的双烯丙基化和烯烃部分的(过度)还原。































 京公网安备 11010802027423号
京公网安备 11010802027423号