Advanced Powder Technology ( IF 4.2 ) Pub Date : 2022-05-05 , DOI: 10.1016/j.apt.2022.103597
Yongjie Liu 1 , Fupeng He 1 , Qingqing Hu 1 , Qingyun Huang 2 , Xuyang Liu 1 , Zhixiong You 1, 3 , Guibao Qiu 1, 3 , Xuewei Lv 1, 3
|
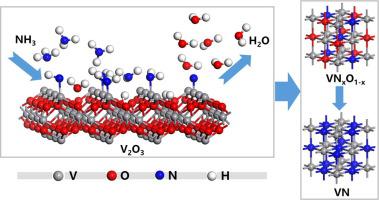
Vanadium nitride (VN) has a wide range of application because of its excellent properties, which include high hardness, outstanding wear resistance, and good electrical conductivity. This study investigated the mechanism for the reduction and nitridation of V2O3 with clean ammonia gas, using both experimental and density functional theory (DFT) studies. The experimental results indicated that V2O3 could easily be converted to VN in an ammonia atmosphere at 500–800 °C. The reaction pathway to form VN was V2O3 → VNxO1–x → VN. Increasing the reaction temperature was conducive to an increase in the N content of VNxO1–x. A DFT study systematically revealed the adsorption of NHx and H on the V2O3 (0001) surface. The results showed that with the dissociation of NH3, its adsorption energy on the surface of vanadium oxide became higher. The whole reaction process could be divided into NH3 decomposition on the surface and the formation of H2O(g). Both of these were endothermic reactions, and the reaction step of generating H2O(g) needed a higher temperature. The bonding of V3c–N facilitated the desorption of O3c atoms to form H2O(g), which explained why VN can be prepared by the reaction of NH3 and V2O3.
中文翻译:

洁净氨气还原和氮化V2O3的相、微观结构演化和周期密度泛函理论研究
氮化钒(VN)由于具有高硬度、出色的耐磨性和良好的导电性等优异性能而具有广泛的应用。本研究使用实验和密度泛函理论 (DFT) 研究,研究了用清洁氨气还原和氮化 V 2 O 3的机理。实验结果表明,在 500-800 ℃的氨气气氛中,V 2 O 3可以很容易地转化为 VN。形成VN的反应途径是V 2 O 3 → VN x O 1– x → VN。提高反应温度有利于增加VN的N含量x O 1– x。DFT 研究系统地揭示了 NH x和 H 在 V 2 O 3 (0001) 表面上的吸附。结果表明,随着NH 3的解离,其在氧化钒表面的吸附能变高。整个反应过程可分为NH 3在表面的分解和H 2 O(g)的形成。这两种反应都是吸热反应,产生H 2 O(g)的反应步骤需要更高的温度。V 3c -N的键合促进O 3c原子解吸形成H 2O(g),这解释了为什么可以通过NH 3和V 2 O 3的反应制备VN 。

































 京公网安备 11010802027423号
京公网安备 11010802027423号