当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
9-epi-Artemisinin – How a Single Stereo Center Affects Chemical Reactivity under Reductive Conditions
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2022-05-04 , DOI: 10.1002/hlca.202100230 Daniel Kweku Anokwah 1 , Pascal Furet 2 , Regis Denay 2 , Dorina Kotoni 3 , Dominique Bixel 3 , Thomas Allmendinger 3
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2022-05-04 , DOI: 10.1002/hlca.202100230 Daniel Kweku Anokwah 1 , Pascal Furet 2 , Regis Denay 2 , Dorina Kotoni 3 , Dominique Bixel 3 , Thomas Allmendinger 3
Affiliation
|
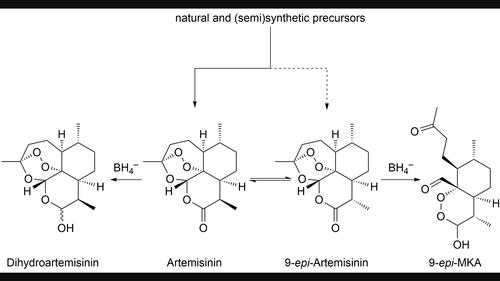
9-epi-Artemisinin occurs as a minor by-product when artemisinin is obtained from natural or semi-synthetic sources. The preparation of β-artemether from artemisinin by reduction with sodium borohydride proceeds normally even when the 9-epimer is present. However, the 9-epimer undergoes rearrangement to peroxy acetals. The results are ascribed to the different stabilities of the corresponding dihydro-intermediates due to steric hindrance as suggested by quantum mechanical calculations.
中文翻译:

9-epi-Artemisinin – 单个立体中心如何影响还原条件下的化学反应性
当青蒿素从天然或半合成来源获得时,9 - epi-青蒿素作为次要副产物出现。即使存在 9-差向异构体,通过用硼氢化钠还原由青蒿素制备 β-蒿甲醚也能正常进行。然而,9-差向异构体经历重排成过氧缩醛。结果归因于量子力学计算表明的由于空间位阻导致的相应二氢中间体的不同稳定性。
更新日期:2022-05-04
中文翻译:

9-epi-Artemisinin – 单个立体中心如何影响还原条件下的化学反应性
当青蒿素从天然或半合成来源获得时,9 - epi-青蒿素作为次要副产物出现。即使存在 9-差向异构体,通过用硼氢化钠还原由青蒿素制备 β-蒿甲醚也能正常进行。然而,9-差向异构体经历重排成过氧缩醛。结果归因于量子力学计算表明的由于空间位阻导致的相应二氢中间体的不同稳定性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号