Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2022-05-04 , DOI: 10.1016/j.jhazmat.2022.129049
Na Chen 1 , Ying Zhao 1 , Meiqi Li 1 , Xiaobing Wang 1 , Xing Peng 1 , Hongwei Sun 1 , Lizhi Zhang 1
|
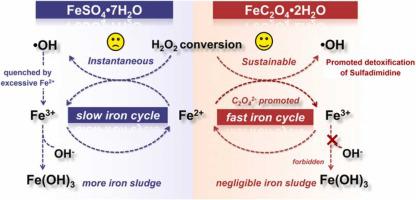
Safe treatment of antibiotics requires efficient removal of both antibiotics and their degraded intermediates. In this study, we demonstrate that FeC2O4•2H2O enables the more sustainable conversion of H2O2 to •OH than commonly used FeSO4•7H2O, promoting the detoxification of a typical antibiotic sulfadimidine. It was found that the FeC2O4/H2O2 system could completely degrade 250 mg L−1 of sulfadimidine within 40 min at pH 3.0, along with decreasing the contents of chemical oxygen demand and total organic carbon by 295.0 and 33.5 mg L−1, respectively, more efficient than those in a classical Fenton system (FeSO4/H2O2). Analysis of sulfadimidine degraded intermediates and toxicity evaluation suggested that the FeC2O4/H2O2 treatment could more effectively decrease the overall toxicity of the sulfadimidine solution than the FeSO4/H2O2 counterpart. The sustainability of FeC2O4•2H2O in H2O2 conversion to •OH was attributed to its controlled release of Fe2+ into the solution to prevent the quenching of •OH by excessive Fe2+, as well as the simultaneous release of C2O42− to complex with Fe2+ and Fe3+, which could inhibit iron sludge formation and accelerate Fe3+/Fe2+ redox cycle. This study provides a promising Fenton system for the safe treatment of antibiotics and sheds light on the potential of FeC2O4•2H2O in environmental remediation.
中文翻译:

FeC2O4•2H2O 能够可持续地将过氧化氢转化为羟基自由基,从而促进磺胺嘧啶的矿化和解毒
抗生素的安全处理需要有效去除抗生素及其降解的中间体。在本研究中,我们证明与常用的 FeSO 4 •7H 2 O 相比,FeC 2 O 4 •2H 2 O 能够更可持续地将 H 2 O 2转化为• OH ,从而促进典型抗生素磺胺嘧啶的解毒。发现FeC 2 O 4 /H 2 O 2体系可以完全降解250 mg L -1在 pH 3.0 条件下,40 分钟内完成磺胺脒,同时化学需氧量和总有机碳含量分别降低 295.0 和 33.5 mg L -1,比经典 Fenton 体系(FeSO 4 /H 2 O 2)。磺胺嘧啶降解中间体的分析和毒性评价表明,与FeSO 4 /H 2 O 2对应物相比,FeC 2 O 4 /H 2 O 2处理可以更有效地降低磺胺嘧啶溶液的总体毒性。FeC 2 O 4 •2H的可持续性H 2 O 2 中的2 O转化为• OH的原因在于其将 Fe 2+控制释放到溶液中以防止过量的 Fe 2+对• OH 的猝灭,以及同时释放 C 2 O 4 2-与Fe 2+和Fe 3+络合,可抑制铁泥形成,加速Fe 3+ /Fe 2+氧化还原循环。该研究为安全治疗抗生素提供了一个很有前景的 Fenton 系统,并揭示了 FeC 2 O 4 •2H 2的潜力O 在环境修复中。

































 京公网安备 11010802027423号
京公网安备 11010802027423号