当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering P450 Monooxygenases for Highly Regioselective and Active p-Hydroxylation of m-Alkylphenols
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-05-05 , DOI: 10.1021/acscatal.1c06011 Ren-Jie Li 1, 2 , Kaiyuan Tian 1 , Xirui Li 1 , Anand Raghavendra Gaikaiwari 1 , Zhi Li 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-05-05 , DOI: 10.1021/acscatal.1c06011 Ren-Jie Li 1, 2 , Kaiyuan Tian 1 , Xirui Li 1 , Anand Raghavendra Gaikaiwari 1 , Zhi Li 1, 2
Affiliation
|
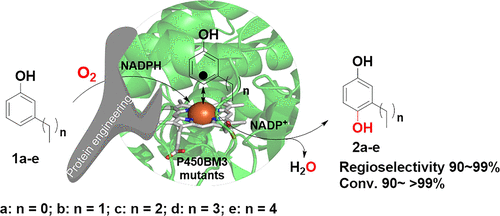
Regioselective hydroxylations of aromatic compounds are useful reactions but often lack appropriate catalysts. Here a group of P450BM3 mutants (R47I/A82F/A328F, R47L/Y51F/F87V/L188P/I401P, R47I/Y51F/F87V, R47L/Y51F/F87V/L181Q/L188P/I401P, and R47I/F87V/L188P) were developed as unique catalysts for the p-hydroxylation of m-alkylphenols 1a–e with high regioselectivity (91–99%) and conversion (95–99%) to produce the corresponding useful and valuable m-alkylbenzene-1,4-diols 2a–e, respectively. The mutated hydroxylases were developed by protein engineering of P450BM3 monooxygenase via site-directed mutagenesis based on designed mutations to reshape the substrate binding pocket and access channel. Several engineered P450BM3 mutants showed good catalytic efficiency (kcat/KM of 234–381 mM–1 min–1) for the p-hydroxylations of m-alkylphenols 1a–e, respectively. Molecular docking and simulation gave some insights into the structure-based understanding of the enhanced regioselectivity and activity for the developed P450BM3 mutants, including the shorter distance between heme-oxygen atom and C4-carbon (p-position) of substrates than the wild-type enzyme in the catalytic pockets. Preparative biohydroxylations of m-alkylphenols 1a–e were demonstrated by using E. coli cells coexpressing individual P450BM3 mutants and glucose dehydrogenase GDH, giving high-yielding synthesis of useful and valuable m-alkylbenzene-1,4-diols 2a–e.
中文翻译:

工程 P450 单加氧酶用于间烷基酚的高度区域选择性和活性对羟基化
芳族化合物的区域选择性羟基化反应是有用的反应,但通常缺乏合适的催化剂。这里开发了一组 P450BM3 突变体(R47I/A82F/A328F、R47L/Y51F/F87V/L188P/I401P、R47I/Y51F/F87V、R47L/Y51F/F87V/L181Q/L188P/I401P 和 R47I/F87V/L188P)作为具有高区域选择性 (91–99%) 和转化率 (95–99%) 的间烷基苯酚1a – e对羟基化的独特催化剂,以生产相应的有用和有价值的间烷基苯-1,4-二醇2a – e, 分别。突变的羟化酶是通过 P450BM3 单加氧酶的蛋白质工程开发的,通过基于设计突变的定点诱变来重塑底物结合袋和进入通道。几个工程化的 P450BM3 突变体对间烷基酚 1a - e 的对羟基化表现出良好的催化效率(k cat / K M为234–381 mM –1 min –1), 分别。分子对接和模拟对基于结构的理解对开发的 P450BM3 突变体的增强区域选择性和活性提供了一些见解,包括血红素氧原子和底物的 C4 碳(p位)之间的距离比野生型更短催化口袋中的酶。通过使用共表达单个 P450BM3 突变体和葡萄糖脱氢酶 GDH 的大肠杆菌细胞证明了间烷基酚 1a-e 的制备性生物羟基化,从而高产地合成了有用且有价值的间烷基苯-1,4-二醇2a - e。
更新日期:2022-05-05
中文翻译:

工程 P450 单加氧酶用于间烷基酚的高度区域选择性和活性对羟基化
芳族化合物的区域选择性羟基化反应是有用的反应,但通常缺乏合适的催化剂。这里开发了一组 P450BM3 突变体(R47I/A82F/A328F、R47L/Y51F/F87V/L188P/I401P、R47I/Y51F/F87V、R47L/Y51F/F87V/L181Q/L188P/I401P 和 R47I/F87V/L188P)作为具有高区域选择性 (91–99%) 和转化率 (95–99%) 的间烷基苯酚1a – e对羟基化的独特催化剂,以生产相应的有用和有价值的间烷基苯-1,4-二醇2a – e, 分别。突变的羟化酶是通过 P450BM3 单加氧酶的蛋白质工程开发的,通过基于设计突变的定点诱变来重塑底物结合袋和进入通道。几个工程化的 P450BM3 突变体对间烷基酚 1a - e 的对羟基化表现出良好的催化效率(k cat / K M为234–381 mM –1 min –1), 分别。分子对接和模拟对基于结构的理解对开发的 P450BM3 突变体的增强区域选择性和活性提供了一些见解,包括血红素氧原子和底物的 C4 碳(p位)之间的距离比野生型更短催化口袋中的酶。通过使用共表达单个 P450BM3 突变体和葡萄糖脱氢酶 GDH 的大肠杆菌细胞证明了间烷基酚 1a-e 的制备性生物羟基化,从而高产地合成了有用且有价值的间烷基苯-1,4-二醇2a - e。






























 京公网安备 11010802027423号
京公网安备 11010802027423号