当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct and efficient synthesis of tetrasubstituted allenyl organothiophosphates from propargylic alcohols under catalyst- and additive-free conditions
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-05-04 , DOI: 10.1039/d2qo00455k Yuxing Zhang 1 , Sha Du 1 , Tao Yang 1 , Fengyan Jin 1 , Jingyi Zhou 1 , Banpeng Cao 1 , Zhi-Jie Mao 1 , Xian-Rong Song 1 , Qiang Xiao 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-05-04 , DOI: 10.1039/d2qo00455k Yuxing Zhang 1 , Sha Du 1 , Tao Yang 1 , Fengyan Jin 1 , Jingyi Zhou 1 , Banpeng Cao 1 , Zhi-Jie Mao 1 , Xian-Rong Song 1 , Qiang Xiao 1
Affiliation
|
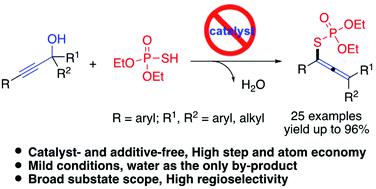
An environment-friendly approach that affords tetrasubstituted allenyl organothiophosphates containing highly congested carbon centers from easily prepared propargylic alcohols and phosphorothioic acids [(RO)2P(O)SH] with water as the only by-product is developed. The reaction is carried out by an in situ dehydrative cross-coupling process involving the allenyl carbocation intermediate and followed by nucleophilic attack to achieve high product distribution. In addition, the reaction occurred in the presence of (EtO)2P(O)SH, and no ligand, additive or additional acid promoter was needed. It is noted that (EtO)2P(O)SH acts not only as an acid promoter but also as a nucleophile. Moreover, a variety of propargylic alcohols bearing electron-rich and electron-withdrawing groups were well tolerated to generate various tetrasubstituted allenyl organothiophosphates in moderate to excellent yields under mild conditions.
中文翻译:

在无催化剂和无添加剂条件下,由炔丙醇直接高效合成四取代丙二烯基有机硫代磷酸酯
开发了一种环境友好的方法,该方法可以从容易制备的炔丙醇和硫代磷酸 [(RO) 2 P(O)SH] 中获得含有高度拥挤碳中心的四取代丙二烯基有机硫代磷酸盐,而水是唯一的副产物。该反应通过原位脱水交叉偶联过程进行,涉及烯基碳正离子中间体,然后进行亲核攻击以实现高产物分布。此外,该反应在 (EtO) 2 P(O)SH 存在下进行,不需要配体、添加剂或额外的酸促进剂。注意到 (EtO) 2P(O)SH 不仅作为酸促进剂,而且作为亲核试剂。此外,多种带有富电子和吸电子基团的炔丙醇具有良好的耐受性,可在温和条件下以中等至优异的产率生成各种四取代的烯基有机硫代磷酸酯。
更新日期:2022-05-04
中文翻译:

在无催化剂和无添加剂条件下,由炔丙醇直接高效合成四取代丙二烯基有机硫代磷酸酯
开发了一种环境友好的方法,该方法可以从容易制备的炔丙醇和硫代磷酸 [(RO) 2 P(O)SH] 中获得含有高度拥挤碳中心的四取代丙二烯基有机硫代磷酸盐,而水是唯一的副产物。该反应通过原位脱水交叉偶联过程进行,涉及烯基碳正离子中间体,然后进行亲核攻击以实现高产物分布。此外,该反应在 (EtO) 2 P(O)SH 存在下进行,不需要配体、添加剂或额外的酸促进剂。注意到 (EtO) 2P(O)SH 不仅作为酸促进剂,而且作为亲核试剂。此外,多种带有富电子和吸电子基团的炔丙醇具有良好的耐受性,可在温和条件下以中等至优异的产率生成各种四取代的烯基有机硫代磷酸酯。































 京公网安备 11010802027423号
京公网安备 11010802027423号