Nano Research ( IF 9.5 ) Pub Date : 2022-05-02 , DOI: 10.1007/s12274-022-4352-0 Ying Liu 1 , Qianqian Xu 1 , Lihang Chen 1 , Changhua Song 1 , Qiwei Yang 1, 2 , Zhiguo Zhang 1, 2 , Dan Lu 1 , Yiwen Yang 1, 2 , Qilong Ren 1, 2 , Zongbi Bao 1, 2
|
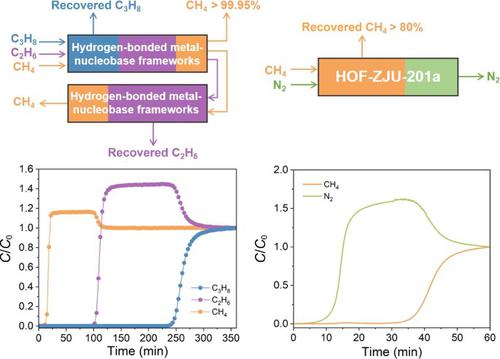
The separation of light hydrocarbons, including C2H6 and C3H8, is essential to natural gas upgrading. Meanwhile, N2 removal from CH4 is also crucial to concentrating low-quality coalbed methane, but the adsorption process is challenging because of the close kinetic diameter. This work reports two hydrogen-bonded metal-nucleobase frameworks (HOF-ZJU-201 and HOF-ZJU-202) capable of efficiently separating C3H8/CH4, C2H6/CH4, and CH4/N2. Due to strong affinity for C3H8 and C2H6, the low-pressure capacity for C3H8 (5 kPa) and C2H6 (10 kPa) of HOF-ZJU-201a exceeds most adsorbents. The ideal adsorbed solution theory (IAST) selectivity of C3H8/CH4 and C2H6/CH4 is 119 and 45 at ambient conditions. According to density functional theory calculations, surface polarization environments formed by electron-rich anions and electron-deficient purine heterocyclic rings contribute to the selective capture of C3H8 and C2H6 with greater polarizability. Furthermore, the high CH4 adsorption capacity (1.73 mmol/g for HOF-ZJU-201a and 1.50 mmol/g for HOF-ZJU-202a at 298 K and 1.0 bar) and excellent CH4/N2 selectivity (6.0 for HOF-ZJU-201 at 298 K), as well as dynamic breakthrough experiments of binary CH4/N2 gas mixture implied their efficacy in the concentration of low-quality coalbed methane.
中文翻译:

用于从甲烷和甲烷/氮气分离中高选择性捕获乙烷/丙烷的氢键金属核碱基框架
轻烃(包括 C 2 H 6和 C 3 H 8)的分离对于天然气提质至关重要。同时,从 CH 4中脱除N 2对浓缩劣质煤层气也至关重要,但由于动力学直径接近,吸附过程具有挑战性。这项工作报告了两种能够有效分离 C 3 H 8 /CH 4、C 2 H 6 /CH 4和 CH 4 /N 2的氢键金属核碱基骨架(HOF-ZJU-201 和 HOF-ZJU-202). 由于对 C 3的强亲和力H 8和C 2 H 6 , HOF-ZJU-201a对C 3 H 8 (5 kPa)和C 2 H 6 (10 kPa)的低压容量超过了大多数吸附剂。在环境条件下,C 3 H 8 /CH 4和 C 2 H 6 /CH 4的理想吸附溶液理论 (IAST) 选择性分别为 119 和 45。根据密度泛函理论计算,富电子阴离子和缺电子嘌呤杂环形成的表面极化环境有助于选择性捕获C 3 H 8和C2 H 6具有更大的极化率。此外,高 CH 4吸附容量(HOF-ZJU-201a 为 1.73 mmol/g,HOF-ZJU-202a 在 298 K 和 1.0 bar 下为 1.50 mmol/g)和优异的 CH 4 /N 2选择性(HOF-为 6.0 ZJU-201 在 298 K),以及二元 CH 4 /N 2气体混合物的动态突破实验表明它们在浓缩低质量煤层气中的功效。






























 京公网安备 11010802027423号
京公网安备 11010802027423号