当前位置:
X-MOL 学术
›
Mol. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-free Synthesis of 5-Trifluoromethyl-1,2,4-triazoles via elemental sulfur promoted oxidative cyclization of trifluoroacetimidohydrazides with benzylic and aliphatic amines
Molecular Catalysis ( IF 3.9 ) Pub Date : 2022-05-01 , DOI: 10.1016/j.mcat.2022.112336 Shu-Ning Lu 1 , Yue Sun 1 , Jiajun Zhang 1 , Zhengkai Chen 1 , Xiao-Feng Wu 2, 3
中文翻译:

通过元素硫无金属合成 5-三氟甲基-1,2,4-三唑促进三氟乙酰亚胺酰肼与苄胺和脂肪胺的氧化环化
更新日期:2022-05-02
Molecular Catalysis ( IF 3.9 ) Pub Date : 2022-05-01 , DOI: 10.1016/j.mcat.2022.112336 Shu-Ning Lu 1 , Yue Sun 1 , Jiajun Zhang 1 , Zhengkai Chen 1 , Xiao-Feng Wu 2, 3
Affiliation
|
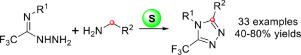
An elemental sulfur-mediated oxidative cyclization of readily available trifluoroacetimidohydrazides and aliphatic amines has been achieved, which provided a direct avenue to structurally diverse 5-trifluoromethyl-1,2,4-triazoles. In this transformation, sulfur acts as a traceless oxidizing agent. A myriad of benzyl amines and tertiary aliphatic amines were tolerated in this protocol. The reaction can be scaled up easily and also been applied to build GlyT1 inhibitor as an example of bio-active molecule.
中文翻译:

通过元素硫无金属合成 5-三氟甲基-1,2,4-三唑促进三氟乙酰亚胺酰肼与苄胺和脂肪胺的氧化环化
已经实现了容易获得的三氟乙酰亚氨基酰肼和脂肪胺的元素硫介导的氧化环化,这为结构多样化的 5-三氟甲基-1,2,4-三唑提供了直接途径。在这种转变中,硫作为一种无痕氧化剂。在该协议中可以耐受大量的苄胺和脂肪族叔胺。该反应可以很容易地放大,也可以用于构建 GlyT1 抑制剂作为生物活性分子的一个例子。































 京公网安备 11010802027423号
京公网安备 11010802027423号