当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of 1,4,2-Dithiazolidines or 1,3-Thiazetidines from 1,1-Dichloro-2-nitroethene and Phenylthiourea Derivatives
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-08-31 00:00:00 , DOI: 10.1021/acs.joc.6b01307 Yian Feng 1 , Minming Zou 1 , Runjiang Song 1 , Xusheng Shao 1 , Zhong Li 1, 2 , Xuhong Qian 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-08-31 00:00:00 , DOI: 10.1021/acs.joc.6b01307 Yian Feng 1 , Minming Zou 1 , Runjiang Song 1 , Xusheng Shao 1 , Zhong Li 1, 2 , Xuhong Qian 1
Affiliation
|
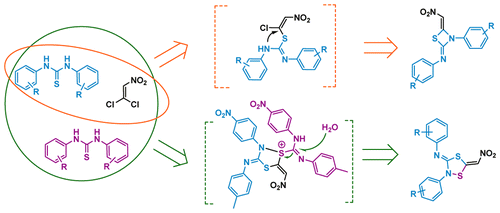
A method for preparation of 1,4,2-dithiazolidine or 1,3-thiazetidine heterocycles was developed by reactions of phenylthioureas with 1,1-dichloro-2-nitroethene. The solvent has a significant influence on the type of product formation. 1,4,2-Dithiazolidines were formed in the aprotic solvent chloroform, while in the protic solvent ethanol, 1,3-thiazetidines were the main products.
中文翻译:

由1,1-二氯-2-硝基乙烯和苯硫脲衍生物形成1,4,2-二噻唑烷或1,3-噻氮烷
通过苯硫脲与1,1-二氯-2-硝基乙烯的反应,开发了一种制备1,4,2-二噻唑烷或1,3-噻氮杂环丁烷杂环的方法。溶剂对产物形成的类型有重大影响。在质子惰性溶剂氯仿中形成1,4,2-二噻唑烷,而在质子惰性溶剂乙醇中,主要产物为1,3-噻唑烷。
更新日期:2016-08-31
中文翻译:

由1,1-二氯-2-硝基乙烯和苯硫脲衍生物形成1,4,2-二噻唑烷或1,3-噻氮烷
通过苯硫脲与1,1-二氯-2-硝基乙烯的反应,开发了一种制备1,4,2-二噻唑烷或1,3-噻氮杂环丁烷杂环的方法。溶剂对产物形成的类型有重大影响。在质子惰性溶剂氯仿中形成1,4,2-二噻唑烷,而在质子惰性溶剂乙醇中,主要产物为1,3-噻唑烷。











































 京公网安备 11010802027423号
京公网安备 11010802027423号