当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
anti-Selective Borylstannylation of Alkynes with (o-Phenylenediaminato)borylstannanes by a Radical Mechanism
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-04-29 , DOI: 10.1002/anie.202201883
Kensuke Suzuki 1 , Naoki Sugihara 1 , Yoshihiro Nishimoto 1, 2 , Makoto Yasuda 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-04-29 , DOI: 10.1002/anie.202201883
Kensuke Suzuki 1 , Naoki Sugihara 1 , Yoshihiro Nishimoto 1, 2 , Makoto Yasuda 1, 2
Affiliation
|
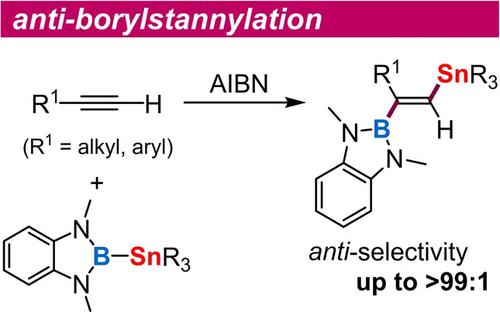
The first anti-borylstannylation of alkynes has been achieved by a radical mechanism with (o-phenylenediaminato)borylstannanes. This anti-addition manner is in contrast to the syn-selectivity in transition metal-catalyzed borylstannylation. 1-Boryl-2-stannylalkenes, which were applicable to sequential cross-coupling, were obtained with excellent regio- and stereoselectivity. The mechanism was revealed by DFT studies.
中文翻译:

炔烃与(邻苯二氨基)硼基锡烷的抗选择性硼基锡烷基化反应
炔烃的第一个抗硼基锡烷化是通过使用(邻苯二氨基)硼基锡烷的自由基机制实现的。这种反加成方式与过渡金属催化的硼烷基化反应中的顺选择性相反。获得了适用于顺序交叉偶联的 1-Boryl-2-stannylalkenes,具有优异的区域选择性和立体选择性。DFT研究揭示了该机制。
更新日期:2022-04-29
中文翻译:

炔烃与(邻苯二氨基)硼基锡烷的抗选择性硼基锡烷基化反应
炔烃的第一个抗硼基锡烷化是通过使用(邻苯二氨基)硼基锡烷的自由基机制实现的。这种反加成方式与过渡金属催化的硼烷基化反应中的顺选择性相反。获得了适用于顺序交叉偶联的 1-Boryl-2-stannylalkenes,具有优异的区域选择性和立体选择性。DFT研究揭示了该机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号