当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Crystalline Structure on the Catalytic Hydrolysis of Cellulose in Subcritical Water
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-04-27 , DOI: 10.1021/acssuschemeng.1c08703 Yue Liu 1 , Hongqiao Fu 1 , Wei Zhang 1 , Haichao Liu 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-04-27 , DOI: 10.1021/acssuschemeng.1c08703 Yue Liu 1 , Hongqiao Fu 1 , Wei Zhang 1 , Haichao Liu 1
Affiliation
|
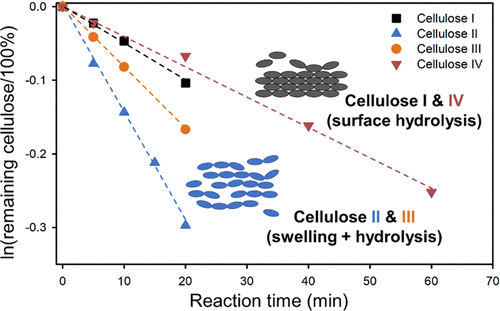
Depolymerization of cellulose, the most abundant biomass in nature, is a critical step for its catalytic conversion to fuels and chemicals. While cleavage of its glycosidic bond by acid hydrolysis is the rate-determining step to depolymerize cellulose, disrupting its robust crystalline structure is equally important. In this work, we examined the hydrolysis of cellulose of four different crystalline allomorphs, i.e., I, II, III, and IV, with respect to the conversion rate, change in the crystalline structure, and the degree of crystallinity and polymerization during the reaction. Independent of their crystalline structure, the four cellulose samples converted following the first-order reaction kinetics with no essential influence on the product selectivity. However, the rate constants were largely different and decreased in the following sequence: cellulose II > III > I > IV. The high rate of cellulose II is caused by its higher reaction probability, as reflected by its preexponential factor, which is several orders of magnitude higher than that for the other cellulose samples, which overcompensated its high apparent activation energy. It is found that cellulose I and IV undergo surface reactions at 478–508 K, whereas cellulose II and III swell at the reaction temperatures, which allows the hydrolysis reaction to occur in the whole swollen regions, leading to higher accessibility of the glycosidic bond to the H+ catalyst and consequently higher conversion rates. These findings provide the mechanistic basis for an alternative strategy to enhance the efficacy in depolymerization of cellulose via tuning of crystalline phases.
中文翻译:

晶体结构对亚临界水中纤维素催化水解的影响
纤维素是自然界中最丰富的生物质,其解聚是其催化转化为燃料和化学品的关键步骤。虽然通过酸水解裂解其糖苷键是解聚纤维素的速率决定步骤,但破坏其坚固的晶体结构同样重要。在这项工作中,我们研究了四种不同结晶同种异形体(即 I、II、III 和 IV)的纤维素水解的转化率、晶体结构的变化以及反应过程中的结晶度和聚合度。 . 与它们的晶体结构无关,四种纤维素样品按照一级反应动力学进行转化,对产物选择性没有本质影响。然而,速率常数有很大不同,并按以下顺序减少:纤维素 II > III > I > IV。纤维素 II 的高速率是由其较高的反应概率引起的,这反映在其指前因子上,该因子比其他纤维素样品高几个数量级,这过度补偿了其高表观活化能。发现纤维素 I 和 IV 在 478-508 K 下发生表面反应,而纤维素 II 和 III 在反应温度下膨胀,这使得水解反应发生在整个膨胀区域,导致糖苷键更容易接近H 这过度补偿了其高表观活化能。发现纤维素 I 和 IV 在 478-508 K 下发生表面反应,而纤维素 II 和 III 在反应温度下膨胀,这使得水解反应发生在整个膨胀区域,导致糖苷键更容易接近H 这过度补偿了其高表观活化能。发现纤维素 I 和 IV 在 478-508 K 下发生表面反应,而纤维素 II 和 III 在反应温度下膨胀,这使得水解反应发生在整个膨胀区域,导致糖苷键更容易接近H+催化剂,因此转化率更高。这些发现为通过调整晶相来提高纤维素解聚功效的替代策略提供了机械基础。
更新日期:2022-04-27
中文翻译:

晶体结构对亚临界水中纤维素催化水解的影响
纤维素是自然界中最丰富的生物质,其解聚是其催化转化为燃料和化学品的关键步骤。虽然通过酸水解裂解其糖苷键是解聚纤维素的速率决定步骤,但破坏其坚固的晶体结构同样重要。在这项工作中,我们研究了四种不同结晶同种异形体(即 I、II、III 和 IV)的纤维素水解的转化率、晶体结构的变化以及反应过程中的结晶度和聚合度。 . 与它们的晶体结构无关,四种纤维素样品按照一级反应动力学进行转化,对产物选择性没有本质影响。然而,速率常数有很大不同,并按以下顺序减少:纤维素 II > III > I > IV。纤维素 II 的高速率是由其较高的反应概率引起的,这反映在其指前因子上,该因子比其他纤维素样品高几个数量级,这过度补偿了其高表观活化能。发现纤维素 I 和 IV 在 478-508 K 下发生表面反应,而纤维素 II 和 III 在反应温度下膨胀,这使得水解反应发生在整个膨胀区域,导致糖苷键更容易接近H 这过度补偿了其高表观活化能。发现纤维素 I 和 IV 在 478-508 K 下发生表面反应,而纤维素 II 和 III 在反应温度下膨胀,这使得水解反应发生在整个膨胀区域,导致糖苷键更容易接近H 这过度补偿了其高表观活化能。发现纤维素 I 和 IV 在 478-508 K 下发生表面反应,而纤维素 II 和 III 在反应温度下膨胀,这使得水解反应发生在整个膨胀区域,导致糖苷键更容易接近H+催化剂,因此转化率更高。这些发现为通过调整晶相来提高纤维素解聚功效的替代策略提供了机械基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号