Molecular Catalysis ( IF 3.9 ) Pub Date : 2022-04-26 , DOI: 10.1016/j.mcat.2022.112323
Haipeng Chen 1 , Ningning Ma 1 , Chenwei Wang 1 , Chenlei Liu 1 , Jiamiao Shen 1 , Youjiao Wang 1 , Gao Xu 1 , Qingfeng Yang 2 , Xun Feng 1
|
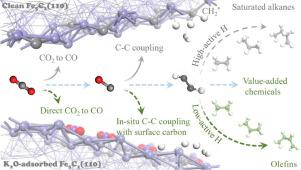
The activation of reactant molecules on catalyst surface plays critical roles in the catalytic reaction process. The activation of CO2 and H2 molecules on clean and K2O-adsorbed Fe5C2(110) were comparatively investigated via density functional theory (DFT) calculations to disclose the promoting effect of K2O on hydrogenation of CO2 to olefins. DFT calculations suggest that the adsorption of K2O helps to direct activation of CO2 to CO species, and then promote in-situ C C* coupling of CO with surface carbon of χ-Fe5C2. The Fe-H bond from H2 dissociation on K2O-adsorbed Fe5C2(110) can lower the activity of H species, by which helps to avoid the over-hydrogenation of olefins to saturated alkanes. This study partially reveals the promoting effect of K2O on the activation of reactant molecules on χ-Fe5C2 surface, which is helpful to design new catalysts for CO2 conversion to value-added chemicals.
C* coupling of CO with surface carbon of χ-Fe5C2. The Fe-H bond from H2 dissociation on K2O-adsorbed Fe5C2(110) can lower the activity of H species, by which helps to avoid the over-hydrogenation of olefins to saturated alkanes. This study partially reveals the promoting effect of K2O on the activation of reactant molecules on χ-Fe5C2 surface, which is helpful to design new catalysts for CO2 conversion to value-added chemicals.
中文翻译:

洞察 CO2 和 H2 在 K2O 吸附 Fe5C2(110) 上用于烯烃生产的活化:密度泛函理论研究
催化剂表面反应物分子的活化在催化反应过程中起着至关重要的作用。通过密度泛函理论(DFT)计算对比研究了CO 2和H 2分子在清洁和K 2 O吸附的Fe 5 C 2 (110)上的活化作用,揭示了K 2 O对CO 2加氢生成的促进作用。烯烃。DFT计算表明,K 2 O的吸附有助于将CO 2直接活化为CO物种,进而促进CO与χ-Fe 5 C 2表面碳的原位C C*偶联 . K 2 O吸附的Fe 5 C 2 (110)上的H 2解离产生的Fe-H键可以降低H物种的活性,这有助于避免烯烃过度氢化成饱和烷烃。本研究部分揭示了K 2 O对χ-Fe 5 C 2表面反应物分子活化的促进作用,有助于设计用于CO 2转化为增值化学品的新型催化剂。
. K 2 O吸附的Fe 5 C 2 (110)上的H 2解离产生的Fe-H键可以降低H物种的活性,这有助于避免烯烃过度氢化成饱和烷烃。本研究部分揭示了K 2 O对χ-Fe 5 C 2表面反应物分子活化的促进作用,有助于设计用于CO 2转化为增值化学品的新型催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号