Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2022-04-26 , DOI: 10.1016/j.jssc.2022.123163 B.P. Sobolev 1
|
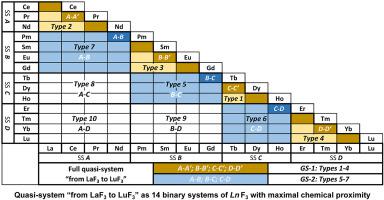
The combination of 15 RF3 (R – rare earth elements – REEs without ScF3 and YF3) into one system “from LaF3 to LuF3” we call a full quasi-system (QS). It consists of 14 serially linked particular systems (PartSs): LaF3–CeF3; CeF3-PrF3; PrF3-NdF3; NdF3-PmF3; PmF3-SmF3; SmF3-EuF3; EuF3-GdF3; GdF3-TbF3; TbF3-DyF3; DyF3-HoF3; HoF3-ErF3; ErF3–TmF3; TmF3-YbF3; YbF3-LuF3. The atomic numbers (Z) of cations from 57 (La) to 71 (Lu) and the temperature (T) are axies of QS. The conditions of including RF3 in PartSs is the neighborhood of R (Ln - lanthanides) positions in Periodic Table of Chemical Elements and |ΔZ| = 1. This securities of maximum chemical proximity (СhProx) of RF3's components. The QS obeys the phase rule, principles of continuity and conformity. Full QS contains all signs of chemical RF3's interactions identified in 34 studied LnF3-Ln’F3 systems. These signs are dependent of ΔZ: perfect and limited isomorphism and two varieties (peritectic and eutectic) of structural true morphotropic transformations (MTs) of phases. Modifications of LnF3 forms the solid solutions (ss) Ln1-xLn'xF3 with average Zav=(1-x)NLn + x(N+1)Ln’ (N from 57 to 71). These ss reveal the lanthanide contraction (LC) in the regions |ΔZav|<1. Full QS is an individual sub-level of chemical classification of LnF3-Ln’F3 systems. It is a tool for studies the chemical interactions LnF3, analyzing fine consequences of LC, creating a special system of Ln3+ ionic radii for LnF3, studies of density anomalies (negative thermal expansion), prediction of phase diagrams unexplored systems RF3-R’F3 and other problems of rare earth trifluorides chemical family.
中文翻译:

完整的准系统“从 LaF3 到 LuF3”,由 14 个三氟化镧系元素的二元系统组合而成,具有最大的化学接近性
将 15 个R F 3 ( R –稀土元素–不含 ScF 3和 YF 3的REE ) 组合成一个“从 LaF 3到 LuF 3 ”的系统,我们称之为全准系统( QS )。它由 14 个串联的特定系统( PartS ) 组成:LaF 3 –CeF 3;CeF 3 -PrF 3;PrF 3 -NdF 3;NdF 3 -PmF 3;PmF 3 -SmF3;SmF 3 -EuF 3;EuF 3 -GdF 3;GdF 3 -TbF 3;TbF 3 -DyF 3;DyF 3 -HoF 3;HoF 3 -ErF 3;ErF 3 –TmF 3;TmF 3 -YbF 3;YbF 3 -LuF 3。从 57 (La) 到 71 (Lu) 的阳离子的原子序数( Z ) 和温度 ( T ) 是 QS 的轴。包含RF 3的条件在 PartSs 是化学元素周期表中R ( Ln - 镧系元素) 位置的邻域和 |ΔZ| = 1。R F 3组件的这种最大化学接近度( СhProx ) 的证券。QS 遵循相位规则、连续性和一致性原则。完整的 QS 包含在 34 个研究的Ln F 3 - Ln' F 3系统中确定的化学R F 3相互作用的所有迹象。这些符号依赖于 ΔZ:完美和有限同构以及两个各种(包晶和共晶)结构真正的变态转变(MTs)相。Ln F 3的变型形成固溶体( ss ) Ln 1-x Ln ' x F 3平均Z av =(1- x ) N Ln + x (N+1) Ln ' (N 从 57 到 71 )。这些ss揭示了镧系元素的收缩(LC) 在区域 |ΔZ av |<1。Full QS 是Ln F 3 - Ln ' F 3系统化学分类的单独子级别。它是研究化学相互作用Ln F 3、分析 LC 的精细结果、为Ln F 3创建一个特殊的Ln 3+离子半径系统、研究密度异常(负热膨胀)、预测相图未探索系统的工具射频3 -射频3 _ _三氟化稀土化学家族的其他问题。

































 京公网安备 11010802027423号
京公网安备 11010802027423号