当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Incorporation of Alkylamine into Metal–Organic Frameworks through a Brønsted Acid–Base Reaction for CO2 Capture
ChemSusChem ( IF 7.5 ) Pub Date : 2016-09-01 , DOI: 10.1002/cssc.201600768 Hao Li 1 , Kecheng Wang 1 , Dawei Feng 1 , Ying-Pin Chen 1, 2 , Wolfgang Verdegaal 3 , Hong-Cai Zhou 1, 2
ChemSusChem ( IF 7.5 ) Pub Date : 2016-09-01 , DOI: 10.1002/cssc.201600768 Hao Li 1 , Kecheng Wang 1 , Dawei Feng 1 , Ying-Pin Chen 1, 2 , Wolfgang Verdegaal 3 , Hong-Cai Zhou 1, 2
Affiliation
|
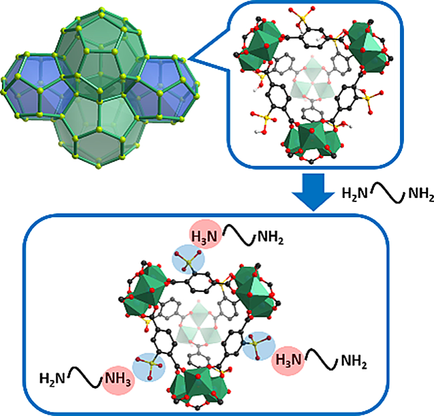
The escalating atmospheric CO2 concentration is one of the most urgent environmental concerns of our age. To effectively capture CO2, various materials have been studied. Among them, alkylamine‐modified metal–organic frameworks (MOFs) are considered to be promising candidates. In most cases, alkylamine molecules are integrated into MOFs through the coordination bonds formed between open metal sites (OMSs) and amine groups. Thus, the alkylamine density, as well as the corresponding CO2 uptake in MOFs, are severely restricted by the density of OMSs. To overcome this limit, other approaches to incorporating alkylamine into MOFs are highly desired. We have developed a new method based on Brønsted acid–base reaction to tether alkylamines into Cr‐MIL‐101‐SO3H for CO2 capture. A systematic optimization of the amine tethering process was also conducted to maximize the CO2 uptake of the modified MOF. Under the optimal amine tethering condition, the obtained tris(2‐aminoethyl)amine‐functionalized Cr‐MIL‐101‐SO3H (Cr‐MIL‐101‐SO3H‐TAEA) has a cyclic CO2 uptake of 2.28 mmol g−1 at 150 mbar and 40 °C, and 1.12 mmol g−1 at 0.4 mbar and 20 °C. The low‐cost starting materials and simple synthetic procedure for the preparation of Cr‐MIL‐101‐SO3H‐TAEA suggest that it has the potential for large‐scale production and practical applications.
中文翻译:

通过布朗斯台德酸碱反应将烷基胺掺入金属有机骨架中以捕集二氧化碳
不断增长的大气中CO 2浓度是我们这个时代最紧迫的环境问题之一。为了有效地捕获CO 2,已经研究了各种材料。其中,烷基胺改性的金属有机骨架(MOF)被认为是很有前途的候选者。在大多数情况下,烷基胺分子通过在开放金属位点(OMS)和胺基团之间形成的配位键整合到MOF中。因此,OMS的密度严重限制了MOF中的烷基胺密度以及相应的CO 2吸收。为了克服该限制,非常需要将烷基胺结合到MOF中的其他方法。我们已经开发了一种基于布朗斯台德酸碱反应将烷基胺拴系到Cr-MIL-101-SO中的新方法3 H用于CO 2捕获。还进行了胺系链工艺的系统优化,以使改性MOF的CO 2吸收最大化。在最佳胺束缚条件下,获得的三(2-氨基乙基)胺官能化的Cr-MIL-101-SO 3 H(Cr-MIL-101-SO 3 H-TAEA)的循环CO 2吸收量为2.28 mmol g -1在150毫巴和40℃,和1.12毫摩尔克-1在0.4毫巴和20℃。制备Cr-MIL-101-SO 3 H-TAEA的低成本起始原料和简单的合成程序表明,它具有大规模生产和实际应用的潜力。
更新日期:2016-09-01
中文翻译:

通过布朗斯台德酸碱反应将烷基胺掺入金属有机骨架中以捕集二氧化碳
不断增长的大气中CO 2浓度是我们这个时代最紧迫的环境问题之一。为了有效地捕获CO 2,已经研究了各种材料。其中,烷基胺改性的金属有机骨架(MOF)被认为是很有前途的候选者。在大多数情况下,烷基胺分子通过在开放金属位点(OMS)和胺基团之间形成的配位键整合到MOF中。因此,OMS的密度严重限制了MOF中的烷基胺密度以及相应的CO 2吸收。为了克服该限制,非常需要将烷基胺结合到MOF中的其他方法。我们已经开发了一种基于布朗斯台德酸碱反应将烷基胺拴系到Cr-MIL-101-SO中的新方法3 H用于CO 2捕获。还进行了胺系链工艺的系统优化,以使改性MOF的CO 2吸收最大化。在最佳胺束缚条件下,获得的三(2-氨基乙基)胺官能化的Cr-MIL-101-SO 3 H(Cr-MIL-101-SO 3 H-TAEA)的循环CO 2吸收量为2.28 mmol g -1在150毫巴和40℃,和1.12毫摩尔克-1在0.4毫巴和20℃。制备Cr-MIL-101-SO 3 H-TAEA的低成本起始原料和简单的合成程序表明,它具有大规模生产和实际应用的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号