当前位置:
X-MOL 学术
›
J. Clin. Lab. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Light-initiated chemiluminescent assay of 17β-estradiol metrological traceability system established by manufacturer according to ISO17511:2020 and basic performance evaluation performed by clinical end-users
Journal of Clinical Laboratory Analysis ( IF 2.6 ) Pub Date : 2022-04-26 , DOI: 10.1002/jcla.24436
Chenyu Shang 1 , Xue Yuan 2 , Haibiao Lin 1 , Dongdong Liu 1 , Xiaoxin Yan 3 , Xinxin Ren 3 , Xiyang Lin 3 , Huang Di 1 , Huiqiang Li 4
Journal of Clinical Laboratory Analysis ( IF 2.6 ) Pub Date : 2022-04-26 , DOI: 10.1002/jcla.24436
Chenyu Shang 1 , Xue Yuan 2 , Haibiao Lin 1 , Dongdong Liu 1 , Xiaoxin Yan 3 , Xinxin Ren 3 , Xiyang Lin 3 , Huang Di 1 , Huiqiang Li 4
Affiliation
|
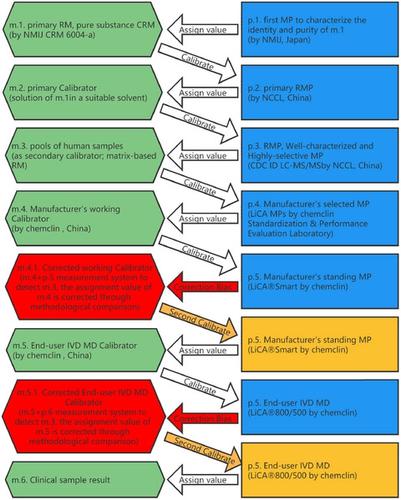
In order to ensure the accuracy of the product, we established 1st model of metrological traceability hierarchy for light-initiated chemiluminescent assay (LICA) of 17β-estradiol (E2) at the manufacturer, based on International Organization for Standardization (ISO) 17511:2020. Moreover, we verified/validated the basic performance (such as matrix effect and long-term stability of end-user IVD MD calibrator, precision, linearity interval, accuracy/ trueness, and detection capability) at the clinical end-user.
中文翻译:

制造商根据ISO17511:2020建立的17β-雌二醇计量溯源系统的光引发化学发光测定和临床最终用户进行的基本性能评估
为确保产品的准确性,我们根据国际标准化组织 (ISO) 17511 在制造商处建立了 17β-雌二醇 (E 2 )光引发化学发光测定 (LICA) 的计量溯源层次模型第一个模型:2020。此外,我们验证/验证了临床最终用户的基本性能(如最终用户 IVD MD 校准器的基质效应和长期稳定性、精度、线性区间、准确度/真实性和检测能力)。
更新日期:2022-04-26
中文翻译:

制造商根据ISO17511:2020建立的17β-雌二醇计量溯源系统的光引发化学发光测定和临床最终用户进行的基本性能评估
为确保产品的准确性,我们根据国际标准化组织 (ISO) 17511 在制造商处建立了 17β-雌二醇 (E 2 )光引发化学发光测定 (LICA) 的计量溯源层次模型第一个模型:2020。此外,我们验证/验证了临床最终用户的基本性能(如最终用户 IVD MD 校准器的基质效应和长期稳定性、精度、线性区间、准确度/真实性和检测能力)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号