当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Aptamer Biosensor Based on ATP-Induced 2D DNA Structure Switching for Rapid and Ultrasensitive Detection of ATP
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-04-26 , DOI: 10.1021/acs.analchem.2c00613 Ying Liu 1 , Lingqi Kong 1 , Hao Li 1 , Ruo Yuan 1 , Yaqin Chai 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-04-26 , DOI: 10.1021/acs.analchem.2c00613 Ying Liu 1 , Lingqi Kong 1 , Hao Li 1 , Ruo Yuan 1 , Yaqin Chai 1
Affiliation
|
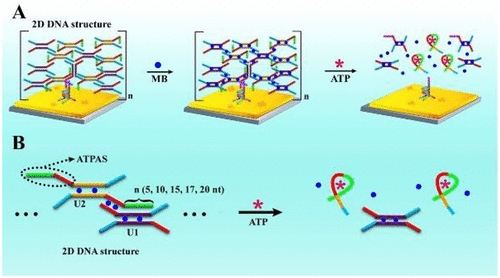
Herein, a two-dimensional (2D) DNA structure with multiple ATP aptamers was elegantly designed to establish an electrochemical biosensor for rapid and sensitive detection of ATP based on ATP-induced structure switching. Concretely, the prepared 2D DNA structure containing numerous ATP aptamers as ATP-specific toehold switches could not only immobilize a large number of methylene blue (MB) for generating a remarkable electrochemical signal, but also greatly increase the local concentration of ATP aptamers to obviously enhance the capture efficiency of ATP. Once the target ATP interacted with the toehold switches, the 2D DNA structure could be sharply collapsed to trigger the burst release of MB from the electrode surface, ultimately resulting in a significantly decreased electrochemical signal for ultrasensitive detection of target ATP over a short period of time. Impressively, by dexterously adjusting the length of the ATP-specific toehold switches to 15-base, optimization of the binding affinity between ATP and the toehold switches was achieved for cutting down the detection time to 30 min and achieving a low detection limit of 0.3 pM, which addressed the shortcoming of time-consuming and poor sensitivity in the previous sensors with a small quantity of ATP aptamers and deficient binding affinity to ATP. Consequently, this strategy opened a promising avenue for ultrasensitive and rapid detection of various biomolecules in biomedical application and disease diagnosis.
中文翻译:

基于 ATP 诱导的二维 DNA 结构转换的电化学适体生物传感器用于 ATP 的快速和超灵敏检测
本文巧妙地设计了具有多个 ATP 适体的二维 (2D) DNA 结构,以建立一种电化学生物传感器,用于基于 ATP 诱导的结构转换快速、灵敏地检测 ATP。具体而言,所制备的含有大量 ATP 适体作为 ATP 特异性立足点开关的 2D DNA 结构不仅可以固定大量亚甲蓝 (MB) 以产生显着的电化学信号,而且可以大大增加 ATP 适体的局部浓度,从而显着增强ATP的捕获效率。一旦目标 ATP 与脚趾开关相互作用,二维 DNA 结构就会急剧塌陷,从而触发电极表面 MB 的爆发释放,最终导致在短时间内超灵敏检测目标 ATP 的电化学信号显着降低。令人印象深刻的是,通过巧妙地将 ATP 特异性立足点开关的长度调整为 15 个碱基,优化了 ATP 和立足点开关之间的结合亲和力,将检测时间缩短至 30 分钟,并实现了 0.3 pM 的低检测限,解决了以往传感器中ATP适配体数量少、与ATP结合亲和力不足的耗时、灵敏度差的缺点。因此,该策略为生物医学应用和疾病诊断中各种生物分子的超灵敏和快速检测开辟了一条有前景的途径。通过巧妙地将 ATP 特异性 toehold 开关的长度调整为 15 个碱基,优化了 ATP 与 toehold 开关之间的结合亲和力,将检测时间缩短至 30 分钟,并实现了 0.3 pM 的低检测限,这解决了以往传感器中ATP适配体数量少、与ATP结合亲和力不足的耗时、灵敏度差的缺点。因此,该策略为生物医学应用和疾病诊断中各种生物分子的超灵敏和快速检测开辟了一条有前景的途径。通过巧妙地将 ATP 特异性 toehold 开关的长度调整为 15 个碱基,优化了 ATP 与 toehold 开关之间的结合亲和力,将检测时间缩短至 30 分钟,并实现了 0.3 pM 的低检测限,这解决了以往传感器中ATP适配体数量少、与ATP结合亲和力不足的耗时、灵敏度差的缺点。因此,该策略为生物医学应用和疾病诊断中各种生物分子的超灵敏和快速检测开辟了一条有前景的途径。解决了以往传感器中ATP适配体数量少、与ATP结合亲和力不足等缺点。因此,该策略为生物医学应用和疾病诊断中各种生物分子的超灵敏和快速检测开辟了一条有前景的途径。解决了以往传感器中ATP适配体数量少、与ATP结合亲和力不足等缺点。因此,该策略为生物医学应用和疾病诊断中各种生物分子的超灵敏和快速检测开辟了一条有前景的途径。
更新日期:2022-04-26
中文翻译:

基于 ATP 诱导的二维 DNA 结构转换的电化学适体生物传感器用于 ATP 的快速和超灵敏检测
本文巧妙地设计了具有多个 ATP 适体的二维 (2D) DNA 结构,以建立一种电化学生物传感器,用于基于 ATP 诱导的结构转换快速、灵敏地检测 ATP。具体而言,所制备的含有大量 ATP 适体作为 ATP 特异性立足点开关的 2D DNA 结构不仅可以固定大量亚甲蓝 (MB) 以产生显着的电化学信号,而且可以大大增加 ATP 适体的局部浓度,从而显着增强ATP的捕获效率。一旦目标 ATP 与脚趾开关相互作用,二维 DNA 结构就会急剧塌陷,从而触发电极表面 MB 的爆发释放,最终导致在短时间内超灵敏检测目标 ATP 的电化学信号显着降低。令人印象深刻的是,通过巧妙地将 ATP 特异性立足点开关的长度调整为 15 个碱基,优化了 ATP 和立足点开关之间的结合亲和力,将检测时间缩短至 30 分钟,并实现了 0.3 pM 的低检测限,解决了以往传感器中ATP适配体数量少、与ATP结合亲和力不足的耗时、灵敏度差的缺点。因此,该策略为生物医学应用和疾病诊断中各种生物分子的超灵敏和快速检测开辟了一条有前景的途径。通过巧妙地将 ATP 特异性 toehold 开关的长度调整为 15 个碱基,优化了 ATP 与 toehold 开关之间的结合亲和力,将检测时间缩短至 30 分钟,并实现了 0.3 pM 的低检测限,这解决了以往传感器中ATP适配体数量少、与ATP结合亲和力不足的耗时、灵敏度差的缺点。因此,该策略为生物医学应用和疾病诊断中各种生物分子的超灵敏和快速检测开辟了一条有前景的途径。通过巧妙地将 ATP 特异性 toehold 开关的长度调整为 15 个碱基,优化了 ATP 与 toehold 开关之间的结合亲和力,将检测时间缩短至 30 分钟,并实现了 0.3 pM 的低检测限,这解决了以往传感器中ATP适配体数量少、与ATP结合亲和力不足的耗时、灵敏度差的缺点。因此,该策略为生物医学应用和疾病诊断中各种生物分子的超灵敏和快速检测开辟了一条有前景的途径。解决了以往传感器中ATP适配体数量少、与ATP结合亲和力不足等缺点。因此,该策略为生物医学应用和疾病诊断中各种生物分子的超灵敏和快速检测开辟了一条有前景的途径。解决了以往传感器中ATP适配体数量少、与ATP结合亲和力不足等缺点。因此,该策略为生物医学应用和疾病诊断中各种生物分子的超灵敏和快速检测开辟了一条有前景的途径。






























 京公网安备 11010802027423号
京公网安备 11010802027423号