Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On-Surface Azide–Alkyne Cycloaddition Reaction: Does It Click with Ruthenium Catalysts?
Langmuir ( IF 3.7 ) Pub Date : 2022-04-26 , DOI: 10.1021/acs.langmuir.2c00100
Tiexin Li 1 , Essam M Dief 1 , Zlatica Kalužná 2, 3 , Melanie MacGregor 4 , Cina Foroutan-Nejad 2, 5 , Nadim Darwish 1
Langmuir ( IF 3.7 ) Pub Date : 2022-04-26 , DOI: 10.1021/acs.langmuir.2c00100
Tiexin Li 1 , Essam M Dief 1 , Zlatica Kalužná 2, 3 , Melanie MacGregor 4 , Cina Foroutan-Nejad 2, 5 , Nadim Darwish 1
Affiliation
|
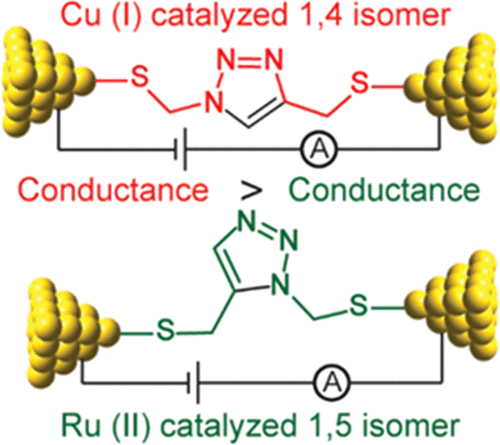
Owing to its simplicity, selectivity, high yield, and the absence of byproducts, the “click” azide–alkyne reaction is widely used in many areas. The reaction is usually catalyzed by copper(I), which selectively produces the 1,4-disubstituted 1,2,3-triazole regioisomer. Ruthenium-based catalysts were later developed to selectively produce the opposite regioselectivity─the 1,5-disubstituted 1,2,3-triazole isomer. Ruthenium-based catalysis, however, remains only tested for click reactions in solution, and the suitability of ruthenium catalysts for surface-based click reactions remains unknown. Also unknown are the electrical properties of the 1,4- and 1,5-regioisomers, and to measure them, both isomers need to be assembled on the electrode surface. Here, we test whether ruthenium catalysts can be used to catalyze surface azide–alkyne reactions to produce 1,5-disubstituted 1,2,3-triazole, and compare their electrochemical properties, in terms of surface coverages and electron transfer kinetics, to those of the compound formed by copper catalysis, 1,4-disubstituted 1,2,3-triazole isomer. Results show that ruthenium(II) complexes catalyze the click reaction on surfaces yielding the 1,5-disubstituted isomer, but the rate of the reaction is remarkably slower than that of the copper-catalyzed reaction, and this is related to the size of the catalyst involved as an intermediate in the reaction. The electron transfer rate constant (ket) for the ruthenium-catalyzed reaction is 30% of that measured for the copper-catalyzed 1,4-isomer. The lower conductivity of the 1,5-isomer is confirmed by performing nonequilibrium Green’s function computations on relevant model systems. These findings demonstrate the feasibility of ruthenium-based catalysis of surface click reactions and point toward an electrical method for detecting the isomers of click reactions.
中文翻译:

表面叠氮化物-炔烃环加成反应:是否与钌催化剂发生点击?
由于其简单、选择性高、产率高且不产生副产物,“点击”叠氮化物-炔反应在许多领域得到广泛应用。该反应通常由铜(I)催化,选择性地产生1,4-二取代的1,2,3-三唑区域异构体。后来开发了基于钌的催化剂来选择性地产生相反的区域选择性——1,5-二取代的1,2,3-三唑异构体。然而,基于钌的催化仍然仅针对溶液中的点击反应进行了测试,并且钌催化剂对于基于表面的点击反应的适用性仍然未知。同样未知的是 1,4- 和 1,5- 区域异构体的电特性,为了测量它们,需要将两种异构体组装在电极表面上。在这里,我们测试了钌催化剂是否可以用于催化表面叠氮化物-炔烃反应来生产1,5-二取代的1,2,3-三唑,并在表面覆盖率和电子转移动力学方面与它们的电化学性能进行比较。铜催化形成的化合物,1,4-二取代的1,2,3-三唑异构体。结果表明,钌(II)配合物催化表面点击反应生成1,5-二取代异构体,但反应速率明显慢于铜催化反应,这与钌(II)配合物的尺寸有关。催化剂作为反应中间体参与反应。钌催化反应的电子转移速率常数 ( k et ) 是铜催化 1,4-异构体测量值的 30%。通过对相关模型系统进行非平衡格林函数计算,证实了 1,5-异构体的较低电导率。这些发现证明了基于钌的表面点击反应催化的可行性,并指出了检测点击反应异构体的电学方法。
更新日期:2022-04-26
中文翻译:

表面叠氮化物-炔烃环加成反应:是否与钌催化剂发生点击?
由于其简单、选择性高、产率高且不产生副产物,“点击”叠氮化物-炔反应在许多领域得到广泛应用。该反应通常由铜(I)催化,选择性地产生1,4-二取代的1,2,3-三唑区域异构体。后来开发了基于钌的催化剂来选择性地产生相反的区域选择性——1,5-二取代的1,2,3-三唑异构体。然而,基于钌的催化仍然仅针对溶液中的点击反应进行了测试,并且钌催化剂对于基于表面的点击反应的适用性仍然未知。同样未知的是 1,4- 和 1,5- 区域异构体的电特性,为了测量它们,需要将两种异构体组装在电极表面上。在这里,我们测试了钌催化剂是否可以用于催化表面叠氮化物-炔烃反应来生产1,5-二取代的1,2,3-三唑,并在表面覆盖率和电子转移动力学方面与它们的电化学性能进行比较。铜催化形成的化合物,1,4-二取代的1,2,3-三唑异构体。结果表明,钌(II)配合物催化表面点击反应生成1,5-二取代异构体,但反应速率明显慢于铜催化反应,这与钌(II)配合物的尺寸有关。催化剂作为反应中间体参与反应。钌催化反应的电子转移速率常数 ( k et ) 是铜催化 1,4-异构体测量值的 30%。通过对相关模型系统进行非平衡格林函数计算,证实了 1,5-异构体的较低电导率。这些发现证明了基于钌的表面点击反应催化的可行性,并指出了检测点击反应异构体的电学方法。







































 京公网安备 11010802027423号
京公网安备 11010802027423号