当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A bioorthogonal chemical reporter for the detection and identification of protein lactylation
Chemical Science ( IF 7.6 ) Pub Date : 2022-04-26 , DOI: 10.1039/d2sc00918h
Yanan Sun 1 , Yanchi Chen 1 , Tao Peng 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2022-04-26 , DOI: 10.1039/d2sc00918h
Yanan Sun 1 , Yanchi Chen 1 , Tao Peng 1, 2
Affiliation
|
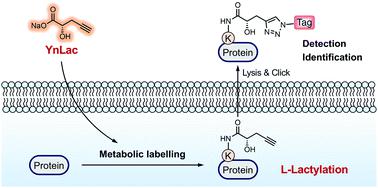
L-Lactylation is a recently discovered post-translational modification occurring on histone lysine residues to regulate gene expression. However, the substrate scope of lactylation, especially that in non-histone proteins, remains unknown, largely due to the limitations of current methods for analyzing lactylated proteins. Herein, we report an alkynyl-functionalized bioorthogonal chemical reporter, YnLac, for the detection and identification of protein lactylation in mammalian cells. Our in-gel fluorescence and chemical proteomic analyses show that YnLac is metabolically incorporated into lactylated proteins and directly labels known lactylated lysines of histones. We further apply YnLac to the proteome-wide profiling of lactylation, revealing many novel modification sites in non-histone proteins for the first time. Moreover, we demonstrate that lactylation of a newly identified substrate protein PARP1 regulates its ADP-ribosylation activity. Our study thus provides a powerful chemical tool for characterizing protein lactylation and greatly expands our understanding of substrate proteins and functions of this new modification.
中文翻译:

用于检测和鉴定蛋白质乳酸化的生物正交化学报告器
大号-乳酸化是最近发现的一种翻译后修饰,发生在组蛋白赖氨酸残基上以调节基因表达。然而,乳酸化的底物范围,尤其是非组蛋白中的底物范围,仍然未知,这主要是由于目前分析乳酸化蛋白质的方法的局限性。在这里,我们报告了一种炔基功能化的生物正交化学报告基因 YnLac,用于检测和鉴定哺乳动物细胞中的蛋白质乳酸化。我们的凝胶内荧光和化学蛋白质组学分析表明,YnLac 在代谢上并入乳酸化蛋白质并直接标记已知的组蛋白乳酸化赖氨酸。我们进一步将 YnLac 应用于蛋白质组范围内的乳酸化分析,首次揭示了非组蛋白中的许多新修饰位点。而且,我们证明了新发现的底物蛋白 PARP1 的乳酸化调节其 ADP 核糖基化活性。因此,我们的研究为表征蛋白质乳酸化提供了一种强大的化学工具,并极大地扩展了我们对底物蛋白和这种新修饰的功能的理解。
更新日期:2022-04-26
中文翻译:

用于检测和鉴定蛋白质乳酸化的生物正交化学报告器
大号-乳酸化是最近发现的一种翻译后修饰,发生在组蛋白赖氨酸残基上以调节基因表达。然而,乳酸化的底物范围,尤其是非组蛋白中的底物范围,仍然未知,这主要是由于目前分析乳酸化蛋白质的方法的局限性。在这里,我们报告了一种炔基功能化的生物正交化学报告基因 YnLac,用于检测和鉴定哺乳动物细胞中的蛋白质乳酸化。我们的凝胶内荧光和化学蛋白质组学分析表明,YnLac 在代谢上并入乳酸化蛋白质并直接标记已知的组蛋白乳酸化赖氨酸。我们进一步将 YnLac 应用于蛋白质组范围内的乳酸化分析,首次揭示了非组蛋白中的许多新修饰位点。而且,我们证明了新发现的底物蛋白 PARP1 的乳酸化调节其 ADP 核糖基化活性。因此,我们的研究为表征蛋白质乳酸化提供了一种强大的化学工具,并极大地扩展了我们对底物蛋白和这种新修饰的功能的理解。







































 京公网安备 11010802027423号
京公网安备 11010802027423号