当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ring-Opening Fluorination of Isoxazoles
Organic Letters ( IF 4.9 ) Pub Date : 2022-04-26 , DOI: 10.1021/acs.orglett.2c01149 Masaaki Komatsuda 1 , Hugo Ohki 1 , Hiroki Kondo 1 , Ayane Suto 1 , Junichiro Yamaguchi 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-04-26 , DOI: 10.1021/acs.orglett.2c01149 Masaaki Komatsuda 1 , Hugo Ohki 1 , Hiroki Kondo 1 , Ayane Suto 1 , Junichiro Yamaguchi 1
Affiliation
|
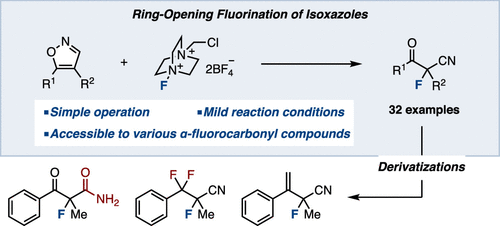
A ring-opening fluorination of isoxazoles has been developed. Upon treatment of isoxazoles with an electrophilic fluorinating agent (Selectfluor), fluorination followed by deprotonation leads to tertiary fluorinated carbonyl compounds. This method features mild reaction conditions, good functional group tolerance, and a simple experimental procedure. Diverse transformations of the resulting α-fluorocyanoketones were also demonstrated, furnishing a variety of fluorinated compounds.
中文翻译:

异恶唑的开环氟化
已经开发了异恶唑的开环氟化。在用亲电子氟化剂 (Selectfluor) 处理异恶唑后,氟化后去质子化产生叔氟化羰基化合物。该方法具有反应条件温和、官能团耐受性好、实验步骤简单等特点。还展示了所得 α-氟氰基酮的多种转化,提供了多种氟化化合物。
更新日期:2022-04-26
中文翻译:

异恶唑的开环氟化
已经开发了异恶唑的开环氟化。在用亲电子氟化剂 (Selectfluor) 处理异恶唑后,氟化后去质子化产生叔氟化羰基化合物。该方法具有反应条件温和、官能团耐受性好、实验步骤简单等特点。还展示了所得 α-氟氰基酮的多种转化,提供了多种氟化化合物。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号