Biomedicine & Pharmacotherapy ( IF 6.9 ) Pub Date : 2022-04-25 , DOI: 10.1016/j.biopha.2022.113031 Filipa Moreira-Silva 1 , Gonçalo Outeiro-Pinho 2 , João Lobo 3 , Rita Guimarães 4 , Vítor M Gaspar 5 , João F Mano 5 , Xabier Agirre 6 , António Pineda-Lucena 6 , Felipe Prosper 6 , Jesus M Paramio 7 , Rui Henrique 8 , Margareta P Correia 9 , Carmen Jerónimo 9
|
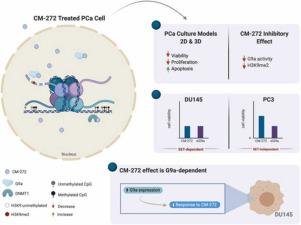
Castration-resistant prostate cancer (CRPC) is an incurable form of prostate cancer (PCa), with DNMT1 and G9a being reported as overexpressed, rendering them highly attractive targets for precision medicine. CM-272 is a dual inhibitor of both methyltransferases’ activity. Herein, we assessed the response of different PCa cell lines to CM-272, in both 2D and 3D models, and explored the molecular mechanisms underlying CM-272 inhibitory effects.
CRPC tissues displayed significantly higher DNMT1, G9a and H3K9me2 expression than localized PCa. In vitro, CM-272 caused a significant decrease in PCa cell viability and proliferation alongside with increased apoptotic levels. We disclose that, under the evaluated dose, CM-272 led to G9a activity inhibition, while not significantly affecting DNMT1 activity. Upon G9a knockdown, DU145 and PC3 showed decreased cell viability. Remarkably, DU145 cells treated with CM-272 or with G9a knockdown displayed no differences in viability, suggesting a SET-dependent mechanism. Contrarily, PC3 cell viability impact was higher in G9a knockdown, compared with CM-272 treatment, suggesting an additional G9a function. Moreover, DU145 cells overexpressing catalytically functional G9a disclosed higher resistance to CM-272 treatment, reinforcing that the drug mechanism of action is dependent on G9a catalytic function.
Importantly, we successfully assembled spheroids from several prostate cell lines. Our results showed that CM-272 retained its anti-tumoral effects in 3D PCa models, leading to a clear reduction in cancer cell survival.
We concluded that inhibition of G9a methyltransferase activity by CM-272 has anti-tumor effect in PCa cells, holding therapeutic potential against CRPC.
中文翻译:

CM-272 抑制 G9a:使用 2D 和 3D 体外模型开发新的去势抵抗性前列腺癌抗肿瘤策略
去势抵抗性前列腺癌 (CRPC) 是一种无法治愈的前列腺癌 (PCa),据报道 DNMT1 和 G9a 过度表达,这使得它们成为精准医学极具吸引力的目标。CM-272 是两种甲基转移酶活性的双重抑制剂。在此,我们在 2D 和 3D 模型中评估了不同 PCa 细胞系对 CM-272 的反应,并探索了 CM-272 抑制作用的分子机制。
CRPC 组织的 DNMT1、G9a 和 H3K9me2 表达明显高于局部 PCa。体外, CM-272 导致 PCa 细胞活力和增殖显着降低,同时凋亡水平增加。我们披露,在评估的剂量下,CM-272 导致 G9a 活性抑制,而不显着影响 DNMT1 活性。在 G9a 敲低后,DU145 和 PC3 显示细胞活力降低。值得注意的是,用 CM-272 或 G9a 敲低处理的 DU145 细胞在活力方面没有差异,表明存在 SET 依赖性机制。相反,与 CM-272 处理相比,G9a 敲低对 PC3 细胞活力的影响更高,这表明 G9a 具有额外的功能。此外,过表达具有催化功能的 G9a 的 DU145 细胞对 CM-272 治疗具有更高的抗性,这表明药物的作用机制依赖于 G9a 催化功能。
重要的是,我们成功地从几个前列腺细胞系中组装了球体。我们的研究结果表明,CM-272 在 3D PCa 模型中保留了其抗肿瘤作用,导致癌细胞存活率明显降低。
我们得出结论,CM-272 对 G9a 甲基转移酶活性的抑制在 PCa 细胞中具有抗肿瘤作用,具有抗 CRPC 的治疗潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号