背景
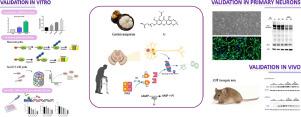
帕金森病(PD)是一种影响患者运动功能的多因素神经退行性疾病。PD 的霍尔标志物是中脑多巴胺能神经元丢失和主要由易聚集蛋白 α-突触核蛋白 (α-syn) 组成的神经元内包涵体的存在。泛素-蛋白酶体系统 (UPS) 是一个多步反应过程,负责 80% 以上的细胞内蛋白质降解。已在 PD 患者的脑组织中观察到 UPS 功能受损。PDE4 抑制剂已被证明可激活 cAMP-PKA 通路并促进阿尔茨海默病模型中的 UPS 活性。α-mangostin 是一种天然的黄酮类化合物,具有广泛的生物活性,例如抗氧化、抗菌和抗肿瘤活性。基于 α-mangostin 的基于结构的优化产生了一种有效的 PDE4 抑制剂 4e。
方法
进行 cAMP 测定以量化样品中的 cAMP 水平。模型 UPS 底物(Ub-G76V-GFP 和 Ub-R-GFP)用于监测 UPS 依赖性活动。通过短肽底物 Suc-LLVY-AMC 研究蛋白酶体活性,蛋白酶体对其的切割增加了荧光敏感性。Tet-on WT、A30P 和 A53T α-syn 诱导型 PC12 细胞和来自 A53T 转基因小鼠的原代小鼠皮质神经元用于评估 4e在体外对 α-syn 的作用。杂合 A53T 转基因小鼠用于评估 4e 对体内α-syn 清除的影响,并通过蛋白质印迹和免疫组织化学进一步验证。
结果
总之,α-mangostin 衍生物 4e,一种 PDE4 抑制剂,有效激活神经元细胞中的 cAMP/PKA 通路,并促进 UPS 活性,这可以通过 UPS 底物 Ub-G76V-GFP 和 Ub-R-GFP 的增强降解来证明,以及如蛋白酶体酶活性升高。有趣的是,4e 以 UPS 依赖的方式显着加速了 PC12 细胞中诱导表达的 WT 和突变体 α-syn 的降解。此外,4e 持续激活原代神经元和 A53T 小鼠大脑中的 PKA,恢复 UPS 抑制并减轻 A53T 小鼠大脑中的 α-syn 积累。
结论
4e 是一种天然化合物衍生的高效 PDE4 抑制剂。我们揭示了它在促进 UPS 活性以降解与 PD 相关的致病蛋白方面的潜在作用。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
α-mangostin derivative 4e as a PDE4 inhibitor promote proteasomal degradation of alpha-synuclein in Parkinson's disease models through PKA activation
Background
Parkinson's disease (PD) is a multi-factorial neurodegenerative disease affecting motor function of patients. The hall markers of PD are dopaminergic neuron loss in the midbrain and the presence of intra-neuronal inclusion bodies mainly composed of aggregation-prone protein alpha-synuclein (α-syn). Ubiquitin-proteasome system (UPS) is a multi-step reaction process responsible for more than 80% intracellular protein degradation. Impairment of UPS function has been observed in the brain tissue of PD patients. PDE4 inhibitors have been shown to activate cAMP-PKA pathway and promote UPS activity in Alzheimer's disease model. α-mangostin is a natural xanthonoid with broad biological activities, such as antioxidant, antimicrobial and antitumour activities. Structure-based optimizations based on α-mangostin produced a potent PDE4 inhibitor, 4e. Herein, we studied whether 4e could promote proteasomal degradation of α-syn in Parkinson's disease models through PKA activation.
Methods
cAMP Assay was conducted to quantify cAMP levels in samples. Model UPS substrates (Ub-G76V-GFP and Ub-R-GFP) were used to monitor UPS-dependent activity. Proteasome activity was investigated by short peptide substrate, Suc-LLVY-AMC, cleavage of which by the proteasome increases fluorescence sensitivity. Tet-on WT, A30P, and A53T α-syn-inducible PC12 cells and primary mouse cortical neurons from A53T transgenic mice were used to evaluate the effect of 4e against α-syn in vitro. Heterozygous A53T transgenic mice were employed to assess the effect of 4e on the clearance of α-syn in vivo, and further validations were applied by western blotting and immunohistochemistry.
Results
Taken together, α-mangostin derivative 4e, a PDE4 inhibitor, efficiently activated the cAMP/PKA pathway in neuronal cells, and promoted UPS activity as evidenced by enhanced degradation of UPS substrate Ub-G76V-GFP and Ub-R-GFP, as well as elevated proteasomal enzyme activity. Interestingly, 4e dramatically accelerated degradation of inducibly-expressed WT and mutant α-syn in PC12 cells, in a UPS dependent manner. Besides, 4e consistently activated PKA in primary neuron and A53T mice brain, restored UPS inhibition and alleviated α-syn accumulation in the A53T mice brain.
Conclusions
4e is a natural compound derived highly potent PDE4 inhibitor. We revealed its potential effect in promoting UPS activity to degrade pathogenic proteins associated with PD.



































 京公网安备 11010802027423号
京公网安备 11010802027423号