当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Mechanism of AlF3 Etching during AlMe3 Exposure: A Thermodynamic and DFT Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-04-25 , DOI: 10.1021/acs.jpcc.2c00158
Xiao Hu 1, 2, 3 , Jörg Schuster 1, 2, 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-04-25 , DOI: 10.1021/acs.jpcc.2c00158
Xiao Hu 1, 2, 3 , Jörg Schuster 1, 2, 3
Affiliation
|
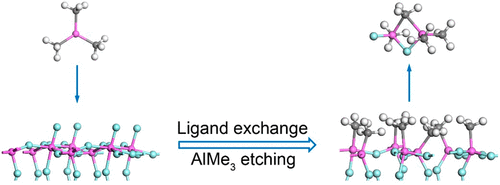
Thermal atomic layer etching (ALE) is a novel approach for the isotropic etching of materials with atomic-level precision. Previous studies have demonstrated the thermal ALE of Al2O3 using sequential, self-limiting reactions with HF and AlMe3 (Me = CH3) as the reactants. It was found that the HF exposure leads to the conversion of Al2O3 to AlF3 and the formation of H2O molecules. The present work aims to investigate the subsequent AlF3 etching during the AlMe3 exposure. On the basis of thermodynamic modeling and density functional theory (DFT) calculations, a two-step mechanism is suggested for the etching of AlF3 by AlMe3. In the first step, an AlF3–xMex (0 < x < 1.5) layer is formed on the surface through the ligand-exchange reaction between AlMe3 and AlF3, with Al2F2Me4 as the main byproduct. The AlF3–xMex surface layer serves as a key intermediate during the etching process. As compared to the original AlF3, AlF3–xMex has a lower stability and can be removed more readily. In the second step, the AlF3–xMex surface layer can be removed via two possible pathways. Pathway I relies on the formation of volatile dimers between AlMe3 and the etched species, while Pathway II is accomplished by the direct decomposition of AlF3–xMex. Due to the higher volatility of the reaction products, Pathway I is found to be more favorable than Pathway II. The feasibility of thermal Al2O3 ALE using HF with alternative precursors SiCl4, TiCl4, and AlClMe2 is evaluated with the above two-step etching mechanism. To realize the AlF3 etching during the precursor exposure, both the formation and removal of the AlF3–xMex surface layer are required to be feasible. Consistent with the experimental results, it is found that only the AlClMe2 precursor meets this requirement and can be considered as a candidate precursor. The presented methodology can be used to guide the development of new precursors for the thermal ALE of Al2O3 and other materials.
中文翻译:

AlMe3 暴露过程中 AlF3 蚀刻的化学机理:热力学和 DFT 研究
热原子层蚀刻 (ALE) 是一种用于以原子级精度对材料进行各向同性蚀刻的新方法。以前的研究已经证明了 Al 2 O 3的热 ALE,使用以 HF 和 AlMe 3 (Me = CH 3 ) 作为反应物的连续自限反应。发现HF暴露导致Al 2 O 3转化为AlF 3并形成H 2 O分子。目前的工作旨在研究AlMe 3过程中随后的 AlF 3蚀刻接触。在热力学建模和密度泛函理论(DFT)计算的基础上,提出了AlMe 3刻蚀AlF 3的两步机制。第一步,通过AlMe 3和AlF 3之间的配体交换反应在表面形成AlF 3– x Me x (0 < x < 1.5)层,其中Al 2 F 2 Me 4为主要副产物。AlF 3– x Me x表面层在蚀刻过程中充当关键中间体。与原来的 AlF 3相比,AlF 3–x Me x稳定性较低,更容易去除。在第二步中,可以通过两种可能的途径去除AlF 3– x Me x表面层。途径 I 依赖于 AlMe 3和蚀刻物质之间挥发性二聚体的形成,而途径 II 是通过 AlF 3– x Me x的直接分解来完成的。由于反应产物的较高挥发性,发现途径 I 比途径 II 更有利。使用 HF 与替代前体 SiCl 4、TiCl 4和 AlClMe 2进行热 Al 2 O 3 ALE 的可行性用上述两步蚀刻机制评估。为了在前驱体曝光期间实现 AlF 3蚀刻,AlF 3– x Me x表面层的形成和去除都需要可行。与实验结果一致,发现只有AlClMe 2前驱体满足这一要求,可以作为候选前驱体。所提出的方法可用于指导 Al 2 O 3和其他材料的热 ALE 新前体的开发。
更新日期:2022-04-25
中文翻译:

AlMe3 暴露过程中 AlF3 蚀刻的化学机理:热力学和 DFT 研究
热原子层蚀刻 (ALE) 是一种用于以原子级精度对材料进行各向同性蚀刻的新方法。以前的研究已经证明了 Al 2 O 3的热 ALE,使用以 HF 和 AlMe 3 (Me = CH 3 ) 作为反应物的连续自限反应。发现HF暴露导致Al 2 O 3转化为AlF 3并形成H 2 O分子。目前的工作旨在研究AlMe 3过程中随后的 AlF 3蚀刻接触。在热力学建模和密度泛函理论(DFT)计算的基础上,提出了AlMe 3刻蚀AlF 3的两步机制。第一步,通过AlMe 3和AlF 3之间的配体交换反应在表面形成AlF 3– x Me x (0 < x < 1.5)层,其中Al 2 F 2 Me 4为主要副产物。AlF 3– x Me x表面层在蚀刻过程中充当关键中间体。与原来的 AlF 3相比,AlF 3–x Me x稳定性较低,更容易去除。在第二步中,可以通过两种可能的途径去除AlF 3– x Me x表面层。途径 I 依赖于 AlMe 3和蚀刻物质之间挥发性二聚体的形成,而途径 II 是通过 AlF 3– x Me x的直接分解来完成的。由于反应产物的较高挥发性,发现途径 I 比途径 II 更有利。使用 HF 与替代前体 SiCl 4、TiCl 4和 AlClMe 2进行热 Al 2 O 3 ALE 的可行性用上述两步蚀刻机制评估。为了在前驱体曝光期间实现 AlF 3蚀刻,AlF 3– x Me x表面层的形成和去除都需要可行。与实验结果一致,发现只有AlClMe 2前驱体满足这一要求,可以作为候选前驱体。所提出的方法可用于指导 Al 2 O 3和其他材料的热 ALE 新前体的开发。

































 京公网安备 11010802027423号
京公网安备 11010802027423号