当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures and chemical bonding of boron-based B12O and B11Au clusters. A counterexample in boronyl chemistry
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-04-25 , DOI: 10.1039/d2cp01277d
Peng-Fei Li 1 , Hua-Jin Zhai 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-04-25 , DOI: 10.1039/d2cp01277d
Peng-Fei Li 1 , Hua-Jin Zhai 1
Affiliation
|
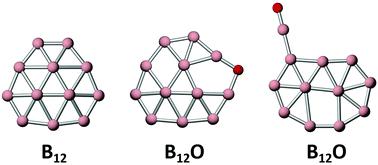
Boron oxide clusters have structural diversity and unique chemical bonding, and recent literature has shown that boronyl complexes dominate boron-rich oxide clusters. A counterexample in boronyl chemistry is presented in this work. Using global structural searches, electronic structure calculations, and chemical bonding analyses, we shall report on the computational design of two boron-based quasi-planar or planar clusters: B12O and B11Au. Contrary to expectation, the B12O cluster has a circular quasi-planar shape with a peripheral B–O–B bridge, which resembles bare B12 cluster. It does not contain a boronyl ligand. The isomeric boronyl complex turns out to be 10.32 kcal mol−1 higher in energy at the single-point CCSD(T) level. In contrast, B11Au cluster behaves normally with an elongated B11 moiety and a terminal Au ligand. Chemical bonding analyses reveal three-fold π/σ aromaticity in circular B12O cluster, including global 6π aromaticity, as well as spatially isolated inner 2σ aromaticity and outer 10σ aromaticity. The three-fold 6π/2σ/10σ aromaticity underlies the stability of B12O cluster. This bonding picture is unknown for bare B12 cluster and its derivatives. The elongated B11Au cluster has conflicting π/σ aromaticity (with 6π versus 8σ electron-counting). The B12O cluster is actually isoelectronic with bare B12 cluster in terms of delocalized π/σ bonding, which inherits the structural and electronic robustness of the latter.
中文翻译:

硼基 B12O 和 B11Au 簇的结构和化学键合。硼基化学的反例
氧化硼簇具有结构多样性和独特的化学键,最近的文献表明硼基配合物在富硼氧化物簇中占主导地位。在这项工作中提出了硼基化学的一个反例。使用全局结构搜索、电子结构计算和化学键分析,我们将报告两个基于硼的准平面或平面簇:B 12 O 和 B 11 Au 的计算设计。与预期相反,B 12 O 星团具有圆形准平面形状,外围有一个 B-O-B 桥,类似于裸 B 12星团。它不含硼基配体。异构硼基络合物结果为 10.32 kcal mol -1在单点 CCSD(T) 水平上的能量更高。相比之下,B 11 Au 簇的行为正常,具有拉长的 B 11部分和末端 Au 配体。化学键合分析揭示了圆形 B 12 O 簇中的三倍 π/σ 芳香性,包括全局 6π 芳香性,以及空间孤立的内部 2σ 芳香性和外部 10σ 芳香性。三倍的6π/2σ/10σ芳香性是B 12 O簇稳定性的基础。对于裸 B 12星团及其衍生物,这种结合图是未知的。拉长的 B 11 Au 簇具有相互矛盾的 π/σ 芳香性(6π对8σ 电子计数)。B 12O簇实际上与裸B 12簇在离域π/σ键合方面是等电子的,继承了后者的结构和电子鲁棒性。
更新日期:2022-04-25
中文翻译:

硼基 B12O 和 B11Au 簇的结构和化学键合。硼基化学的反例
氧化硼簇具有结构多样性和独特的化学键,最近的文献表明硼基配合物在富硼氧化物簇中占主导地位。在这项工作中提出了硼基化学的一个反例。使用全局结构搜索、电子结构计算和化学键分析,我们将报告两个基于硼的准平面或平面簇:B 12 O 和 B 11 Au 的计算设计。与预期相反,B 12 O 星团具有圆形准平面形状,外围有一个 B-O-B 桥,类似于裸 B 12星团。它不含硼基配体。异构硼基络合物结果为 10.32 kcal mol -1在单点 CCSD(T) 水平上的能量更高。相比之下,B 11 Au 簇的行为正常,具有拉长的 B 11部分和末端 Au 配体。化学键合分析揭示了圆形 B 12 O 簇中的三倍 π/σ 芳香性,包括全局 6π 芳香性,以及空间孤立的内部 2σ 芳香性和外部 10σ 芳香性。三倍的6π/2σ/10σ芳香性是B 12 O簇稳定性的基础。对于裸 B 12星团及其衍生物,这种结合图是未知的。拉长的 B 11 Au 簇具有相互矛盾的 π/σ 芳香性(6π对8σ 电子计数)。B 12O簇实际上与裸B 12簇在离域π/σ键合方面是等电子的,继承了后者的结构和电子鲁棒性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号