Redox Biology ( IF 10.7 ) Pub Date : 2022-04-21 , DOI: 10.1016/j.redox.2022.102317 Shumin Ouyang 1 , Huaxuan Li 1 , Linlin Lou 1 , Qiuyao Huang 1 , Zhenhua Zhang 1 , Jianshan Mo 1 , Min Li 2 , Jiaye Lu 1 , Kai Zhu 3 , Yunjie Chu 3 , Wen Ding 1 , Jianzheng Zhu 1 , Ziyou Lin 1 , Lin Zhong 4 , Junjian Wang 1 , Peibin Yue 5 , James Turkson 5 , Peiqing Liu 1 , Yuanxiang Wang 1 , Xiaolei Zhang 1
|
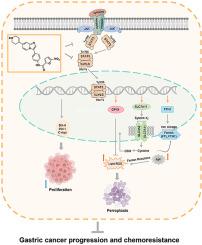
Chemotherapy is still one of the principal treatments for gastric cancer, but the clinical application of 5-FU is limited by drug resistance. Here, we demonstrate that ferroptosis triggered by STAT3 inhibition may provide a novel opportunity to explore a new effective therapeutic strategy for gastric cancer and chemotherapy resistance. We find that ferroptosis negative regulation (FNR) signatures are closely correlated with the progression and chemoresistance of gastric cancer. FNR associated genes (GPX4, SLC7A11, and FTH1) and STAT3 are upregulated in 5-FU resistant cells and xenografts. Further evidence demonstrates that STAT3 binds to consensus DNA response elements in the promoters of the FNR associated genes (GPX4, SLC7A11, and FTH1) and regulates their expression, thereby establishing a negative STAT3-ferroptosis regulatory axis in gastric cancer. Genetic inhibition of STAT3 activity triggers ferroptosis through lipid peroxidation and Fe2+ accumulation in gastric cancer cells. We further develop a potent and selective STAT3 inhibitor, W1131, which demonstrates significant anti-tumor effects in gastric cancer cell xenograft model, organoids model, and patient-derived xenografts (PDX) model partly by inducing ferroptosis, thus providing a new candidate compound for advanced gastric cancer. Moreover, targeting the STAT3-ferroptosis circuit promotes ferroptosis and restores sensitivity to chemotherapy. Our finding reveals that STAT3 acts as a key negative regulator of ferroptosis in gastric cancer through a multi-pronged mechanism and provides a new therapeutic strategy for advanced gastric cancer and chemotherapy resistance.
中文翻译:

抑制 STAT3-ferroptosis 负调节轴抑制肿瘤生长并减轻胃癌的化学抗性
化疗仍然是胃癌的主要治疗方法之一,但5-FU的临床应用受到耐药性的限制。在这里,我们证明了由 STAT3 抑制引发的铁死亡可能为探索胃癌和化疗耐药的新有效治疗策略提供新的机会。我们发现铁死亡负调控(FNR)特征与胃癌的进展和化疗耐药密切相关。FNR 相关基因(GPX4、SLC7A11 和 FTH1)和 STAT3 在 5-FU 抗性细胞和异种移植物中上调。进一步的证据表明,STAT3 与 FNR 相关基因(GPX4、SLC7A11 和 FTH1)启动子中的共有 DNA 反应元件结合并调节它们的表达,从而在胃癌中建立负性STAT3-铁死亡调节轴。STAT3活性的遗传抑制通过脂质过氧化和Fe触发铁死亡2+在胃癌细胞中的积累。我们进一步开发了一种强效和选择性的 STAT3 抑制剂 W1131,该抑制剂在胃癌细胞异种移植模型、类器官模型和患者来源的异种移植 (PDX) 模型中表现出显着的抗肿瘤作用,部分通过诱导铁死亡,从而为晚期胃癌。此外,靶向 STAT3 铁死亡电路可促进铁死亡并恢复对化疗的敏感性。我们的研究结果表明,STAT3通过多管齐下的机制作为胃癌铁死亡的关键负调节因子,为晚期胃癌和化疗耐药提供了新的治疗策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号