当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Detection of the Sulfhydryl Groups in Proteins with Slow Hydrogen Exchange Rates and Determination of Their Proton/Deuteron Fractionation Factors Using the Deuterium-Induced Effects on the13CβNMR Signals
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-05-05 , DOI: 10.1021/ja101205j Mitsuhiro Takeda 1 , JunGoo Jee 1 , Tsutomu Terauchi 1 , Masatsune Kainosho 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-05-05 , DOI: 10.1021/ja101205j Mitsuhiro Takeda 1 , JunGoo Jee 1 , Tsutomu Terauchi 1 , Masatsune Kainosho 1
Affiliation
|
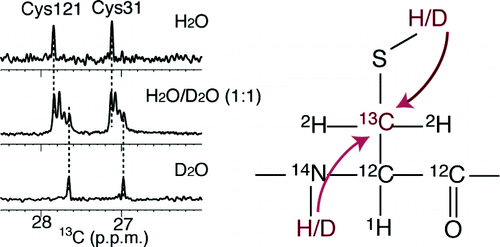
A method for identifying cysteine (Cys) residues with sulfhydryl (SH) groups exhibiting slow hydrogen exchange rates has been developed for proteins in aqueous media. The method utilizes the isotope shifts of the C(beta) chemical shifts induced by the deuteration of the SH groups. The 18.2 kDa E. coli peptidyl prolyl cis-trans isomerase b (EPPIb), which was selectively labeled with [3-(13)C;3,3-(2)H(2)]Cys, showed much narrower line widths for the (13)C(beta) NMR signals, as compared to those of the proteins labeled with either [3-(13)C]Cys or (3R)-[3-(13)C;3-(2)H]Cys. The (13)C(beta) signals of the two Cys residues of EPPIb, i.e. Cys-31 and Cys-121, labeled with [3-(13)C;3,3-(2)H(2)]Cys, split into four signals in H(2)O/D(2)O (1:1) at 40 degrees C and pH 7.5, indicating that the exchange rates of the side-chain SH's and the backbone amides are too slow to average the chemical shift differences of the (13)C(beta) signals, due to the two- and three-bond isotope shifts. By virtue of the well-separated signals, the proton/deuteron fractional factors for both the SH and amide groups of the two Cys residues in EPPIb could be directly determined, as approximately 0.4-0.5 for [SD]/[SH] and 0.9-1.0 for [ND]/[NH], by the relative intensities of the NMR signals for the isotopomers. The proton NOE's of the two slowly exchanging SH's were clearly identified in the NOESY spectra and were useful for the determining the local structure of EPPIb around the Cys residues.
中文翻译:

检测氢交换率低的蛋白质中的巯基并利用氘诱导的 13 Cβ NMR 信号确定其质子/氘分馏因子
已经开发了一种用于识别具有氢交换速率缓慢的巯基 (SH) 基团的半胱氨酸 (Cys) 残基的方法,用于水性介质中的蛋白质。该方法利用由 SH 基团的氘化引起的 C(β) 化学位移的同位素位移。用 [3-(13)C;3,3-(2)H(2)]Cys 选择性标记的 18.2 kDa 大肠杆菌肽基脯氨酰顺反异构酶 b (EPPIb) 显示出更窄的线宽(13)C(beta) NMR 信号,与用 [3-(13)C]Cys 或 (3R)-[3-(13)C;3-(2)H] 标记的蛋白质相比半胱氨酸。EPPIb的两个Cys残基即Cys-31和Cys-121的(13)C(β)信号,用[3-(13)C;3,3-(2)H(2)]Cys标记,在 40 摄氏度和 pH 7.5 下,在 H(2)O/D(2)O (1:1) 中分成四个信号,表明侧链 SH' 的交换率 由于二键和三键同位素位移,s 和主链酰胺太慢而无法平均 (13)C(beta) 信号的化学位移差异。凭借分离良好的信号,可以直接确定 EPPIb 中两个 Cys 残基的 SH 和酰胺基团的质子/氘核分数因子,对于 [SD]/[SH] 约为 0.4-0.5,对于 [SD]/[SH] 约为 0.9- [ND]/[NH] 为 1.0,由同位素异构体的 NMR 信号的相对强度确定。两个缓慢交换的 SH 的质子 NOE 在 NOESY 光谱中被清楚地识别出来,可用于确定 Cys 残基周围的 EPPIb 局部结构。可以直接确定 EPPIb 中两个 Cys 残基的 SH 和酰胺基团的质子/氘核分数因子,[SD]/[SH] 约为 0.4-0.5,[ND]/[NH] 约为 0.9-1.0 ,由同位素异构体的 NMR 信号的相对强度。两个缓慢交换的 SH 的质子 NOE 在 NOESY 光谱中被清楚地识别出来,可用于确定 Cys 残基周围的 EPPIb 局部结构。可以直接确定 EPPIb 中两个 Cys 残基的 SH 和酰胺基团的质子/氘核分数因子,[SD]/[SH] 约为 0.4-0.5,[ND]/[NH] 约为 0.9-1.0 ,由同位素异构体的 NMR 信号的相对强度。两个缓慢交换的 SH 的质子 NOE 在 NOESY 光谱中被清楚地识别出来,可用于确定 Cys 残基周围的 EPPIb 局部结构。
更新日期:2010-05-05
中文翻译:

检测氢交换率低的蛋白质中的巯基并利用氘诱导的 13 Cβ NMR 信号确定其质子/氘分馏因子
已经开发了一种用于识别具有氢交换速率缓慢的巯基 (SH) 基团的半胱氨酸 (Cys) 残基的方法,用于水性介质中的蛋白质。该方法利用由 SH 基团的氘化引起的 C(β) 化学位移的同位素位移。用 [3-(13)C;3,3-(2)H(2)]Cys 选择性标记的 18.2 kDa 大肠杆菌肽基脯氨酰顺反异构酶 b (EPPIb) 显示出更窄的线宽(13)C(beta) NMR 信号,与用 [3-(13)C]Cys 或 (3R)-[3-(13)C;3-(2)H] 标记的蛋白质相比半胱氨酸。EPPIb的两个Cys残基即Cys-31和Cys-121的(13)C(β)信号,用[3-(13)C;3,3-(2)H(2)]Cys标记,在 40 摄氏度和 pH 7.5 下,在 H(2)O/D(2)O (1:1) 中分成四个信号,表明侧链 SH' 的交换率 由于二键和三键同位素位移,s 和主链酰胺太慢而无法平均 (13)C(beta) 信号的化学位移差异。凭借分离良好的信号,可以直接确定 EPPIb 中两个 Cys 残基的 SH 和酰胺基团的质子/氘核分数因子,对于 [SD]/[SH] 约为 0.4-0.5,对于 [SD]/[SH] 约为 0.9- [ND]/[NH] 为 1.0,由同位素异构体的 NMR 信号的相对强度确定。两个缓慢交换的 SH 的质子 NOE 在 NOESY 光谱中被清楚地识别出来,可用于确定 Cys 残基周围的 EPPIb 局部结构。可以直接确定 EPPIb 中两个 Cys 残基的 SH 和酰胺基团的质子/氘核分数因子,[SD]/[SH] 约为 0.4-0.5,[ND]/[NH] 约为 0.9-1.0 ,由同位素异构体的 NMR 信号的相对强度。两个缓慢交换的 SH 的质子 NOE 在 NOESY 光谱中被清楚地识别出来,可用于确定 Cys 残基周围的 EPPIb 局部结构。可以直接确定 EPPIb 中两个 Cys 残基的 SH 和酰胺基团的质子/氘核分数因子,[SD]/[SH] 约为 0.4-0.5,[ND]/[NH] 约为 0.9-1.0 ,由同位素异构体的 NMR 信号的相对强度。两个缓慢交换的 SH 的质子 NOE 在 NOESY 光谱中被清楚地识别出来,可用于确定 Cys 残基周围的 EPPIb 局部结构。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号