Biomedicine & Pharmacotherapy ( IF 6.9 ) Pub Date : 2022-04-22 , DOI: 10.1016/j.biopha.2022.112988 Kai Hu 1 , Xiaozheng Yuan 1 , Huan He 1 , Hui Zhang 1 , Fengsong Wang 1 , Jing Qiao 1
|
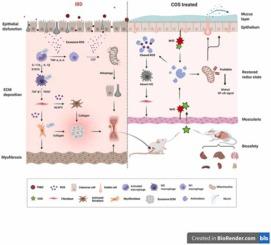
Although extensive development has been made in the treatment of inflammatory bowel disease (IBD), adverse effects and incomplete efficacy of currently used medications are continuous challenge. Accumulated reports on the benefits of chitosan oligosaccharides in intestinal disorders make chitotriose (COS) a breakthrough in the development of new IBD drugs. This study aimed to investigate the biosafety, efficacy and pharmacological mechanisms of COS in the treatment of experimental IBD in compare with the commercial 5-Aminosalicylic acid (5-ASA). In this study, COS effectively relieved active inflammation, restored epithelial function, and reduced intestinal fibrosis. Further investigation demonstrated that COS treatment regulated redox state of the colon tissue by stimulating the transcription factor nuclear factor E2-related factor 2 (Nrf2), increasing production of endogenous antioxidants, and alleviating oxidative stress. The offset of oxidative stress shut down the nuclear factor kappa-B (NF-ĸB) inflammatory pathway, mitophagy of epithelial cells, M2 macrophage polarization in pre-fibrotic inflammation, and myofibroblast activation in intestinal fibrogenesis. In conclusion, COS is a safe and effective therapeutic agent for experimental IBD as a redox regulator. Our results expand the current understanding of the pharmacology of chitosan oligosaccharides for IBD treatment and provides experimental basis for the medicinal development of small molecule carbohydrates.
中文翻译:

壳三糖作为氧化还原调节剂治疗大鼠炎症性肠病的药理机制
尽管炎症性肠病 (IBD) 的治疗已经取得了广泛的发展,但目前使用的药物的副作用和不完全的疗效是持续的挑战。关于壳寡糖在肠道疾病中的益处的累积报道使壳三糖(COS)成为开发IBD新药的突破口。本研究旨在比较 COS 与市售的 5-氨基水杨酸 (5-ASA) 治疗实验性 IBD 的生物安全性、疗效和药理机制。在本研究中,COS 有效缓解活动性炎症,恢复上皮功能,减少肠道纤维化。进一步的研究表明,COS 治疗通过刺激转录因子核因子 E2 相关因子 2 (Nrf2) 来调节结肠组织的氧化还原状态,增加内源性抗氧化剂的产生,并减轻氧化应激。氧化应激的抵消关闭了核因子 kappa-B (NF-ĸB) 炎症通路、上皮细胞的线粒体自噬、纤维化前炎症中的 M2 巨噬细胞极化以及肠纤维化中的肌成纤维细胞活化。总之,COS作为氧化还原调节剂是一种安全有效的实验性IBD治疗剂。我们的研究结果扩展了目前对壳寡糖治疗IBD药理学的认识,并为小分子碳水化合物的药物开发提供实验依据。纤维化前炎症中的 M2 巨噬细胞极化,以及肠纤维化中的肌成纤维细胞活化。总之,COS作为氧化还原调节剂是一种安全有效的实验性IBD治疗剂。我们的研究结果扩展了目前对壳寡糖治疗IBD药理学的认识,并为小分子碳水化合物的药物开发提供实验依据。纤维化前炎症中的 M2 巨噬细胞极化,以及肠纤维化中的肌成纤维细胞活化。总之,COS作为氧化还原调节剂是一种安全有效的实验性IBD治疗剂。我们的研究结果扩展了目前对壳寡糖治疗IBD药理学的认识,并为小分子碳水化合物的药物开发提供实验依据。































 京公网安备 11010802027423号
京公网安备 11010802027423号