当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Evidence for Tunneling and a Hidden Intermediate in the Biosynthesis of Tetrahydrocannabinol
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-04-22 , DOI: 10.1021/jacs.1c11981 Edyta M Greer 1 , Victor Siev 1 , Ayelet Segal 1 , Alexander Greer 2, 3 , Charles Doubleday 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-04-22 , DOI: 10.1021/jacs.1c11981 Edyta M Greer 1 , Victor Siev 1 , Ayelet Segal 1 , Alexander Greer 2, 3 , Charles Doubleday 4
Affiliation
|
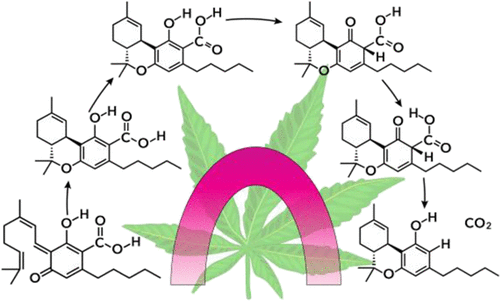
Quantum tunneling is computed for a reaction sequence that models the conversion of the ortho-quinone methide of cannabigerolic acid 1 to the decarboxylated product (−)-trans-Δ9-tetrahydrocannabinol (THC, 3). This calculation is the first to evaluate multidimensional tunneling in this sequence. Computations were carried out with POLYRATE and GAUSSRATE using B3LYP/6-31G(d,p) to examine the mechanism of THC 3 formation. The pentyl chain on THC 3 and its precursors were replaced with a methyl group to compute tunneling contributions to the rates of four separate steps: (i) initial Diels–Alder reaction of the quinone methide with the trisubstituted alkene end-group of the geranyl 1Z-CH3 to give 2Z-CH3, (ii) acid-catalyzed keto–enol tautomerization, which converts 2rZ-CH3 to 4rZ-CH3, (iii) carboxyl rotamerization converting 4rZ-CH3 to 4E-CH3, and (iv) decarboxylation that converts 4E-CH3 to 3-CH3. Tunneling contributions to the rate constants of steps (i)-(iv) are between 19 and 76% at 293 K. In step (ii), nonuniform changes in the zero-point vibrational energy along the reaction path created a shallow minimum in the 0 K free energy. It is a hidden intermediate because it is not a minimum on the potential energy surface and is detectable only when zero-point energy is taken into account along the reaction path. Predicted kinetic isotope effects would be experimentally observable at temperatures that are convenient to use. This is particularly relevant in the decarboxylation stage of the reaction sequence and has important implications because of its role in THC 3 formation.
中文翻译:

四氢大麻酚生物合成中隧道效应和隐藏中间体的计算证据
计算了一个反应序列的量子隧道效应,该反应序列模拟了大麻二酚酸1的邻醌甲基化物向脱羧产物 (-)-反式-Δ 9 - 四氢大麻酚 (THC, 3 ) 的转化。该计算是第一个评估此序列中的多维隧道效应的计算。使用 B3LYP/6-31G(d,p) 对 POLYRATE 和 GAUSSRATE 进行了计算,以检查 THC 3形成的机制。THC 3上的戊基链并将其前体替换为甲基,以计算隧道效应对四个单独步骤的速率的贡献:(i) 醌甲基化物与香叶基1 Z -CH 3的三取代烯烃端基的初始 Diels-Alder 反应,得到2 Z -CH 3, (ii) 酸催化的酮-烯醇互变异构,将2r Z -CH 3转化为4r Z -CH 3,(iii) 羧基旋转异构将4r Z -CH 3转化为4 E -CH 3,和(iv) 转化的脱羧4 E - CH 3至3-CH 3。在 293 K 时,对步骤 (i)-(iv) 的速率常数的隧穿贡献在 19% 和 76% 之间。在步骤 (ii) 中,沿反应路径的零点振动能量的不均匀变化在0 K 自由能。它是一个隐藏的中间体,因为它不是势能表面上的最小值,并且只有在沿反应路径考虑零点能量时才能检测到。预测的动力学同位素效应将在便于使用的温度下通过实验观察到。这与反应序列的脱羧阶段特别相关,并且由于其在 THC 3中的作用而具有重要意义形成。
更新日期:2022-04-22
中文翻译:

四氢大麻酚生物合成中隧道效应和隐藏中间体的计算证据
计算了一个反应序列的量子隧道效应,该反应序列模拟了大麻二酚酸1的邻醌甲基化物向脱羧产物 (-)-反式-Δ 9 - 四氢大麻酚 (THC, 3 ) 的转化。该计算是第一个评估此序列中的多维隧道效应的计算。使用 B3LYP/6-31G(d,p) 对 POLYRATE 和 GAUSSRATE 进行了计算,以检查 THC 3形成的机制。THC 3上的戊基链并将其前体替换为甲基,以计算隧道效应对四个单独步骤的速率的贡献:(i) 醌甲基化物与香叶基1 Z -CH 3的三取代烯烃端基的初始 Diels-Alder 反应,得到2 Z -CH 3, (ii) 酸催化的酮-烯醇互变异构,将2r Z -CH 3转化为4r Z -CH 3,(iii) 羧基旋转异构将4r Z -CH 3转化为4 E -CH 3,和(iv) 转化的脱羧4 E - CH 3至3-CH 3。在 293 K 时,对步骤 (i)-(iv) 的速率常数的隧穿贡献在 19% 和 76% 之间。在步骤 (ii) 中,沿反应路径的零点振动能量的不均匀变化在0 K 自由能。它是一个隐藏的中间体,因为它不是势能表面上的最小值,并且只有在沿反应路径考虑零点能量时才能检测到。预测的动力学同位素效应将在便于使用的温度下通过实验观察到。这与反应序列的脱羧阶段特别相关,并且由于其在 THC 3中的作用而具有重要意义形成。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号