当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity of Nitric Oxide and Nitrosonium Ion with Copper(II/I) Schiff Base Complexes: Mechanistic Aspects of Imine C═N Bond Cleavage and Oxidation of Pyridine-2-aldehyde to Pyridine-2-carboxylic Acid
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-04-22 , DOI: 10.1021/acs.inorgchem.1c04038 Saikat Mishra 1 , Shibaditya Kumar 1 , Anirban Bhandari 1 , Aniruddha Das 1 , Pallav Mondal 1 , Geeta Hundal 2 , Marilyn M Olmstead 3 , Apurba K Patra 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-04-22 , DOI: 10.1021/acs.inorgchem.1c04038 Saikat Mishra 1 , Shibaditya Kumar 1 , Anirban Bhandari 1 , Aniruddha Das 1 , Pallav Mondal 1 , Geeta Hundal 2 , Marilyn M Olmstead 3 , Apurba K Patra 1
Affiliation
|
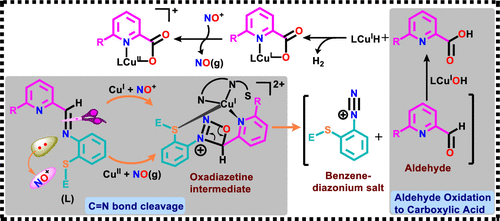
Four Schiff base ligands of the general formulas [6-(R)-2-pyridyl-N-(2′-methylthiophenyl)methylenimine] (RL1) and 6-p-chlorophenyl-2-pyridyl-N-(2′-phenylthiophenyl)methylenimine (RL2), where R = H, Me, p-ClPh, and their bis-ligand copper(II) and copper(I) complexes, 1–4 and 1′–4′, respectively, were synthesized and characterized. The reactivities of 1–4 with nitric oxide (NO) gas and of 1′–4′ with solid NOBF4 (NO+) were examined in dry acetonitrile in the presence and absence of water (H2O). The results revealed that, in the absence of H2O, complexes 1–4 (or 1′–4′) reacts with NO (or NOBF4), leading to imine C═N bond cleavage of both (or one) Schiff base(s) that generates 2 (or 1) equiv of 2-(methyl/phenyl)thiobenzenediazonium perchlorates (5/6) and the corresponding picolaldehyde (RPial) via a copper nitrosyl of a {CuNO}10-type intermediate. In the presence of H2O, the in situ formed RPial get oxidized to the corresponding picolinic acid (RPicH) via an in situ formed LCuIOH intermediate (LCuI + HO-NO → LCuIOH + NO+; L = RL1/RL2/RPic– and νO–H of CuIOH = 3650 cm–1) and subsequently produces, with the aid of NO+ oxidant, the picolinate-ligated copper(II) complexes (i) [(HPic)2Cu] (7), [(MePic)4Cu3(NO3)2]n·H2O (8·H2O), or [(ClPhPic)2Cu] (9) when NO reacts with 1–4 or (ii) [(RPic)CuII(RL1/RL2)]+ when NO+ reacts with 1′–4′. The CuII to CuI reduction of [(RPic)CuII(RL1/RL2)]+ is essential for C═N cleavage of the remaining RL1/RL2 Schiff base; excess NO can do it. The X-ray structures (1, 1′, 3′, 5, 7, and 8) and spectroscopic results revealed the role of CuII/I, NO, NO+, and H2O, shedding light on the mechanism of C═N bond cleavage and the oxidation of pyridine-2-aldehyde to pyridine-2-carboxylic acid. The reaction of 1 with 15NO revealed that the terminal N of the N2+ group of 5 originates from 15NO [ν14N14N– = 2248 cm–1 and ν15N14N– = 2212 cm–1].
中文翻译:

一氧化氮和亚硝基离子与铜 (II/I) 席夫碱配合物的反应性:亚胺 C=N 键断裂和吡啶-2-醛氧化成吡啶-2-羧酸的机理方面
通式 [6-( R )-2-吡啶基-N- (2'-甲基硫苯基)亚甲基亚胺] ( R L1) 和 6-对- 氯苯基-2-吡啶基- N - (2'-) 的四个席夫碱配体苯硫基苯基)亚甲基亚胺 ( R L2),其中 R = H、Me、p -ClPh,以及它们的双配体铜 (II) 和铜 (I) 配合物,分别为1 – 4和1' – 4',被合成并表征。1 – 4与一氧化氮 (NO) 气体的反应性以及1' – 4'与固体 NOBF 4 (NO +) 在存在和不存在水 (H 2 O) 的情况下在无水乙腈中进行检查。结果表明,在没有 H 2 O的情况下,配合物1 – 4(或1' – 4')与 NO(或 NOBF 4)反应,导致两个(或一个)席夫碱的亚胺 C=N 键断裂(s)通过 {CuNO} 10型中间体的亚硝基铜生成 2(或 1)当量的 2-(甲基/苯基)硫代苯重氮高氯酸盐 ( 5 / 6 ) 和相应的吡啶甲醛 ( R Pial)。在 H 2 O的存在下,原位形成R Pial 通过原位形成的 LCu I OH 中间体 (LCu I + HO-NO → LCu I OH + NO + ; L = R L1/ R L2/ R Pic –和 ν )被氧化成相应的吡啶甲酸 ( R PicH) Cu I OH = 3650 cm –1 ) 的O–H并随后在 NO +氧化剂的帮助下生成吡啶配位铜 (II) 配合物 (i) [( H Pic) 2 Cu] ( 7 ), [ ( Me Pic) 4 Cu 3 (NO 3) 2 ] n ·H 2 O ( 8 ·H 2 O),或 [( ClPh Pic) 2 Cu] ( 9 ) 当 NO 与1 – 4或 (ii) [( R Pic)Cu II ( R L1/ R L2)] +当 NO +与1' – 4'反应时。[( R Pic )Cu II ( R L1 / R L2 ) ] +对于剩余的R L1/ R L2 席夫碱的 C =N 裂解是必不可少的;过量的NO可以做到。X射线结构(1、1 '、3'、5、7和8 )和光谱结果揭示了Cu II/I、NO、NO +和H 2 O的作用,揭示了C的机制=N键断裂和吡啶-2-醛氧化成吡啶-2-羧酸。1与15 NO的反应表明,N 2 +基团5的末端 N来源于15NO [ν 14 N 14 N– = 2248 cm –1和 ν 15 N 14 N– = 2212 cm –1 ]。
更新日期:2022-04-22
中文翻译:

一氧化氮和亚硝基离子与铜 (II/I) 席夫碱配合物的反应性:亚胺 C=N 键断裂和吡啶-2-醛氧化成吡啶-2-羧酸的机理方面
通式 [6-( R )-2-吡啶基-N- (2'-甲基硫苯基)亚甲基亚胺] ( R L1) 和 6-对- 氯苯基-2-吡啶基- N - (2'-) 的四个席夫碱配体苯硫基苯基)亚甲基亚胺 ( R L2),其中 R = H、Me、p -ClPh,以及它们的双配体铜 (II) 和铜 (I) 配合物,分别为1 – 4和1' – 4',被合成并表征。1 – 4与一氧化氮 (NO) 气体的反应性以及1' – 4'与固体 NOBF 4 (NO +) 在存在和不存在水 (H 2 O) 的情况下在无水乙腈中进行检查。结果表明,在没有 H 2 O的情况下,配合物1 – 4(或1' – 4')与 NO(或 NOBF 4)反应,导致两个(或一个)席夫碱的亚胺 C=N 键断裂(s)通过 {CuNO} 10型中间体的亚硝基铜生成 2(或 1)当量的 2-(甲基/苯基)硫代苯重氮高氯酸盐 ( 5 / 6 ) 和相应的吡啶甲醛 ( R Pial)。在 H 2 O的存在下,原位形成R Pial 通过原位形成的 LCu I OH 中间体 (LCu I + HO-NO → LCu I OH + NO + ; L = R L1/ R L2/ R Pic –和 ν )被氧化成相应的吡啶甲酸 ( R PicH) Cu I OH = 3650 cm –1 ) 的O–H并随后在 NO +氧化剂的帮助下生成吡啶配位铜 (II) 配合物 (i) [( H Pic) 2 Cu] ( 7 ), [ ( Me Pic) 4 Cu 3 (NO 3) 2 ] n ·H 2 O ( 8 ·H 2 O),或 [( ClPh Pic) 2 Cu] ( 9 ) 当 NO 与1 – 4或 (ii) [( R Pic)Cu II ( R L1/ R L2)] +当 NO +与1' – 4'反应时。[( R Pic )Cu II ( R L1 / R L2 ) ] +对于剩余的R L1/ R L2 席夫碱的 C =N 裂解是必不可少的;过量的NO可以做到。X射线结构(1、1 '、3'、5、7和8 )和光谱结果揭示了Cu II/I、NO、NO +和H 2 O的作用,揭示了C的机制=N键断裂和吡啶-2-醛氧化成吡啶-2-羧酸。1与15 NO的反应表明,N 2 +基团5的末端 N来源于15NO [ν 14 N 14 N– = 2248 cm –1和 ν 15 N 14 N– = 2212 cm –1 ]。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号