Nano Research ( IF 9.5 ) Pub Date : 2022-04-20 , DOI: 10.1007/s12274-022-4299-1
Xin Li 1 , Fengling Zhao 1 , Xiaotong Yang 1 , Qiang Yuan 1, 2 , Ke Xin Yao 3 , Yongfei Li 3 , Jingwei Li 4
|
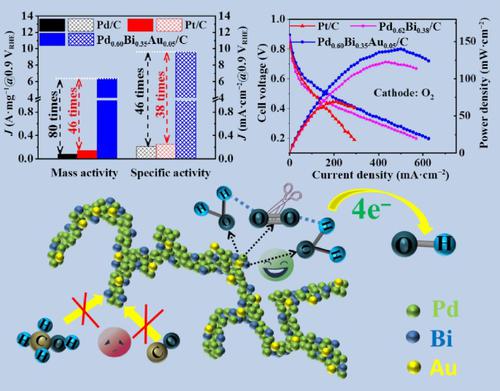
The development of cathode oxygen reduction reaction (ORR) catalysts with high characteristics for practical, direct methanol fuel cells (DMFCs) has continuously increased the attention of researchers. In this work, interface-rich Au-doped PdBi (PdBiAu) branched one-dimensional (1D) alloyed nanochains assembled by sub-6.5 nm particles have been prepared, exhibiting an ORR mass activity (MA) of 6.40 A·mgPd−1 and long-term durability of 5,000 cycles in an alkaline medium. The MA of PdBiAu nanochains is 46 times and 80 times higher than that of commercial Pt/C (0.14 A·mgPt−1) and Pd/C (0.08 A·mgPd−1). The MA of binary PdBi nanochains also reaches 5.71 A·mgPd−1. Notably, the PdBiAu nanochains exhibit high in-situ carbon monoxide poisoning resistance and high methanol tolerance. In actual DMFC device tests, the PdBiAu nanochains enhance power density of 140.1 mW·cm−2 (in O2)/112.4 mW·cm−2 (in air) and durability compared with PdBi nanochains and Pt/C. The analysis of the structure—function relationship indicates that the enhanced performance of PdBiAu nanochains is attributed to integrated functions of surficial defect-rich 1D chain structure, improved charge transfer capability, downshift of the d-band center of Pd, as well as the synergistic effect derived from “Pd-Bi” and/or “Pd-Au” dual active sites.
中文翻译:

富界面 Au 掺杂 PdBi 合金纳米链作为多功能氧还原催化剂提高了直接甲醇燃料电池装置的功率密度和耐用性
用于实用的直接甲醇燃料电池(DMFC)的高性能阴极氧还原反应(ORR)催化剂的开发不断增加研究人员的关注。在这项工作中,制备了由亚 6.5 nm 颗粒组装而成的富界面 Au 掺杂 PdBi (PdBiAu) 分支一维 (1D) 合金纳米链,其 ORR 质量活度 (MA) 为 6.40 A·mg Pd -1在碱性介质中长期耐用 5,000 次。PdBiAu纳米链的MA分别是商业Pt/C(0.14 A·mg Pt -1)和Pd/C(0.08 A·mg Pd -1 )的46倍和80倍。二元PdBi纳米链的MA也达到5.71 A·mg Pd -1. 值得注意的是,PdBiAu 纳米链表现出高原位一氧化碳中毒抗性和高甲醇耐受性。在实际的DMFC器件测试中,与PdBi纳米链和Pt/C相比,PdBiAu纳米链提高了140.1 mW·cm -2 (在O 2中)/112.4 mW·cm -2 (在空气中)的功率密度和耐久性。结构-功能关系分析表明,PdBiAu纳米链的性能增强归因于表面缺陷丰富的一维链结构的综合功能、提高的电荷转移能力、Pd的d带中心下移以及协同作用。源自“Pd-Bi”和/或“Pd-Au”双活性位点的效应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号