当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quaternary Ammonium Ion-Tethered (Ambient-Temperature) HDDA Reactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-04-20 , DOI: 10.1021/jacs.2c00877 Chenlong Zhu 1 , Thomas R Hoye 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-04-20 , DOI: 10.1021/jacs.2c00877 Chenlong Zhu 1 , Thomas R Hoye 1
Affiliation
|
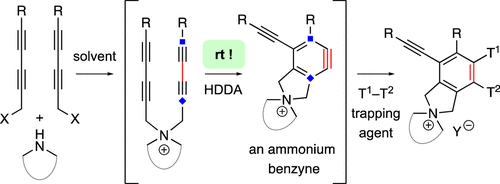
The hexadehydro-Diels–Alder (HDDA) reaction converts a 1,3-diyne bearing a tethered alkyne (the diynophile) into bicyclic benzyne intermediates upon thermal activation. With only a few exceptions, this unimolecular cycloisomerization requires, depending on the nature of the atoms connecting the diyne and diynophile, reaction temperatures of ca. 80–130 °C to achieve a convenient half-life (e.g., 1–10 h) for the reaction. In this report, we divulge a new variant of the HDDA process in which the tether contains a central, quaternized nitrogen atom. This construct significantly lowers the activation barrier for the HDDA cycloisomerization to the benzyne. Moreover, many of the ammonium ion-based, alkyne-containing substrates can be spontaneously assembled, cyclized to benzyne, and trapped in a single-vessel, ambient-temperature operation. DFT calculations provide insights into the origin of the enhanced rate of benzyne formation.
中文翻译:

季铵离子系链(环境温度)HDDA 反应
六脱氢-狄尔斯-桤木 (HDDA) 反应在热活化时将带有束缚炔烃(亲二炔)的 1,3-二炔转化为双环苯炔中间体。除了少数例外,这种单分子环化异构化需要ca的反应温度,具体取决于连接二炔和亲二炔试剂的原子的性质。80–130 °C 以获得方便的半衰期(例如, 1–10 h) 用于反应。在本报告中,我们揭示了 HDDA 过程的一种新变体,其中系链包含一个中心的季铵化氮原子。该构建体显着降低了 HDDA 环化异构化为苯的活化屏障。此外,许多基于铵离子、含炔烃的底物可以自发组装、环化为苯并捕获在单容器、环境温度操作中。DFT 计算提供了对苯炔形成率提高的起源的见解。
更新日期:2022-04-20
中文翻译:

季铵离子系链(环境温度)HDDA 反应
六脱氢-狄尔斯-桤木 (HDDA) 反应在热活化时将带有束缚炔烃(亲二炔)的 1,3-二炔转化为双环苯炔中间体。除了少数例外,这种单分子环化异构化需要ca的反应温度,具体取决于连接二炔和亲二炔试剂的原子的性质。80–130 °C 以获得方便的半衰期(例如, 1–10 h) 用于反应。在本报告中,我们揭示了 HDDA 过程的一种新变体,其中系链包含一个中心的季铵化氮原子。该构建体显着降低了 HDDA 环化异构化为苯的活化屏障。此外,许多基于铵离子、含炔烃的底物可以自发组装、环化为苯并捕获在单容器、环境温度操作中。DFT 计算提供了对苯炔形成率提高的起源的见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号