当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N,N-Dimethylethanesulfonamide as an Electrolyte Solvent Stable for the Positive Electrode Reaction of Aprotic Li–O2 Batteries
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-04-18 , DOI: 10.1021/acsaem.1c03999 Kiho Nishioka 1 , Morihiro Saito 2 , Manai Ono 3 , Shoichi Matsuda 3, 4 , Shuji Nakanishi 1, 3, 5
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-04-18 , DOI: 10.1021/acsaem.1c03999 Kiho Nishioka 1 , Morihiro Saito 2 , Manai Ono 3 , Shoichi Matsuda 3, 4 , Shuji Nakanishi 1, 3, 5
Affiliation
|
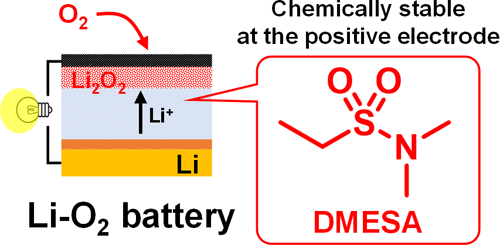
The realization of secondary lithium–oxygen batteries (Li–O2 batteries, LOBs) with large gravimetric energy density requires the development of an innovative electrolyte with high chemical stability that allows the charge–discharge reaction to proceed with low overvoltage. In this study, we evaluated the potential of an electrolyte solvent, N,N-dimethylethanesulfonamide (DMESA) with a sulfonamide functional group, at a current density of 0.4 mA cm–2 and a capacity of 4 mA h cm–2. The voltage at which CO2 was generated during charging was substantially higher than that of a tetraglyme (G4)-based electrolyte with redox mediators, which is one of the standard electrolytes used for LOBs. Experiments using a 13C-containing positive electrode revealed that CO2 generated during charging mainly originated from the decomposition of the positive electrode. The analyses of the charging profile in conjunction with differential electrochemical mass spectrometry suggested the formation of highly degradable lithium peroxide (Li2O2) in the DMESA-based electrolyte. The formation of highly degradable Li2O2 enables a reduction of the charging voltage, leading to further suppression of the electrolyte decomposition.
中文翻译:

N,N-二甲基乙磺酰胺作为非质子 Li-O2 电池正极反应的稳定电解质溶剂
要实现具有大重量能量密度的二次锂-氧电池(Li-O 2电池,LOBs),需要开发一种具有高化学稳定性的创新电解质,使充放电反应能够在低过电压下进行。在本研究中,我们评估了具有磺酰胺官能团的电解质溶剂N , N-二甲基乙磺酰胺 (DMESA) 在电流密度为 0.4 mA cm –2和容量为 4 mA h cm –2下的电势。CO 2的电压充电过程中产生的浓度明显高于具有氧化还原介质的基于四甘醇二甲醚 (G4) 的电解质,后者是用于 LOB 的标准电解质之一。使用含13 C正极的实验表明,充电过程中产生的CO 2主要来源于正极的分解。结合差分电化学质谱法对充电曲线的分析表明,在基于 DMESA 的电解质中形成了高度可降解的过氧化锂 (Li 2 O 2 )。高度可降解Li 2 O 2的形成能够降低充电电压,从而进一步抑制电解质分解。
更新日期:2022-04-18
中文翻译:

N,N-二甲基乙磺酰胺作为非质子 Li-O2 电池正极反应的稳定电解质溶剂
要实现具有大重量能量密度的二次锂-氧电池(Li-O 2电池,LOBs),需要开发一种具有高化学稳定性的创新电解质,使充放电反应能够在低过电压下进行。在本研究中,我们评估了具有磺酰胺官能团的电解质溶剂N , N-二甲基乙磺酰胺 (DMESA) 在电流密度为 0.4 mA cm –2和容量为 4 mA h cm –2下的电势。CO 2的电压充电过程中产生的浓度明显高于具有氧化还原介质的基于四甘醇二甲醚 (G4) 的电解质,后者是用于 LOB 的标准电解质之一。使用含13 C正极的实验表明,充电过程中产生的CO 2主要来源于正极的分解。结合差分电化学质谱法对充电曲线的分析表明,在基于 DMESA 的电解质中形成了高度可降解的过氧化锂 (Li 2 O 2 )。高度可降解Li 2 O 2的形成能够降低充电电压,从而进一步抑制电解质分解。































 京公网安备 11010802027423号
京公网安备 11010802027423号