当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly diastereoselective synthesis of an octahydro-1H-cyclpenta[c]pyridine skeleton via a Pd/Au-relay catalyzed reaction of (Z)-1-iodo-1,6-diene and alkyne
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-04-20 , DOI: 10.1039/d2qo00233g Xiaochen Chi 1 , Tong Xia 1 , Yi Yang 1 , Tong Cao 1 , Daopeng Zhang 1 , Hui Liu 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-04-20 , DOI: 10.1039/d2qo00233g Xiaochen Chi 1 , Tong Xia 1 , Yi Yang 1 , Tong Cao 1 , Daopeng Zhang 1 , Hui Liu 1
Affiliation
|
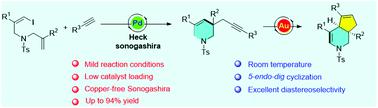
The octahydro-1H-cyclopenta[c]pyridine skeletons exist in a broad spectrum of bioactive natural products, and the development of efficient and convenient protocols to construct this skeleton remains a challenging task. Herein, we report a novel diastereoselective synthesis strategy to produce octahydro-1H-cyclopenta[c]pyridine skeleton from (Z)-1-iodo-1,6-diene and alkyne via Pd/Au-relay catalyzed sequential intramolecular Heck-type cyclization, Sonogashira coupling and 1,5-enyne cyclization. The Heck/Sonogashira sequential coupling was achieved with low palladium catalyst loading without copper to deliver a variety of piperidines bearing 1,5-enyne motifs, which could furnish the octahydro-1H-cyclopenta[c]pyridine derivates with excellent diastereoselectivity (>99.5 : 1) in the presence of IPrAuCl/AgBF4.
中文翻译:

通过 Pd/Au 接力催化 (Z)-1-iodo-1,6-diene 和炔烃反应高度非对映选择性合成八氢-1H-环戊并[c]吡啶骨架
octahydro-1 H -cyclopenta[ c ]pyridine 骨架存在于广泛的生物活性天然产物中,开发高效便捷的方案来构建该骨架仍然是一项具有挑战性的任务。在此,我们报道了一种新的非对映选择性合成策略,通过 ( Z ) -1-iodo-1,6-diene 和炔烃通过Pd/Au-继电器催化顺序分子内 Heck 型环化、Sonogashira 偶联和 1,5-烯炔环化。Heck/Sonogashira 顺序偶联是在不含铜的低钯催化剂负载下实现的,以提供各种带有 1,5-烯炔基序的哌啶,这可以提供具有出色非对映选择性( > 99.5 : 1) 在 IPrAuCl/AgBF 4存在下。
更新日期:2022-04-20
中文翻译:

通过 Pd/Au 接力催化 (Z)-1-iodo-1,6-diene 和炔烃反应高度非对映选择性合成八氢-1H-环戊并[c]吡啶骨架
octahydro-1 H -cyclopenta[ c ]pyridine 骨架存在于广泛的生物活性天然产物中,开发高效便捷的方案来构建该骨架仍然是一项具有挑战性的任务。在此,我们报道了一种新的非对映选择性合成策略,通过 ( Z ) -1-iodo-1,6-diene 和炔烃通过Pd/Au-继电器催化顺序分子内 Heck 型环化、Sonogashira 偶联和 1,5-烯炔环化。Heck/Sonogashira 顺序偶联是在不含铜的低钯催化剂负载下实现的,以提供各种带有 1,5-烯炔基序的哌啶,这可以提供具有出色非对映选择性( > 99.5 : 1) 在 IPrAuCl/AgBF 4存在下。






























 京公网安备 11010802027423号
京公网安备 11010802027423号