European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-04-19 , DOI: 10.1016/j.ejmech.2022.114362 Maria Giulia Nizi 1 , Mirko M Maksimainen 2 , Sudarshan Murthy 2 , Serena Massari 1 , Juho Alaviuhkola 2 , Barbara E Lippok 3 , Sven T Sowa 2 , Albert Galera-Prat 2 , Renata Prunskaite-Hyyryläinen 2 , Bernhard Lüscher 3 , Patricia Korn 3 , Lari Lehtiö 2 , Oriana Tabarrini 1
|
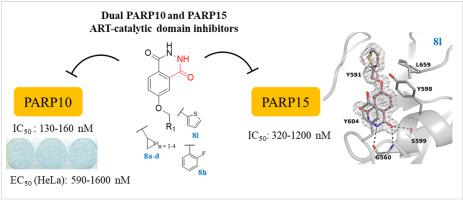
While human poly-ADP-ribose chain generating poly-ARTs, PARP1 and 2 and TNKS1 and 2, have been widely characterized, less is known on the pathophysiological roles of the mono-ADP-ribosylating mono-ARTs, partly due to the lack of selective inhibitors. In this context, we have focused on the development of inhibitors for the mono-ART PARP10, whose overexpression is known to induce cell death. Starting from OUL35 (1) and its 4-(benzyloxy)benzamidic derivative (2) we herein report the design and synthesis of new analogues from which the cyclobutyl derivative 3c rescued cells most efficiently from PARP10 induced apoptosis. Most importantly, we also identified 2,3-dihydrophthalazine-1,4-dione as a new suitable nicotinamide mimicking PARP10 inhibitor scaffold. When it was functionalized with cycloalkyl (8a-c), o-fluorophenyl (8h), and thiophene (8l) rings, IC50 values in the 130–160 nM range were obtained, making them the most potent PARP10 inhibitors reported to date. These compounds also inhibited PARP15 with low micromolar IC50s, but none of the other tested poly- and mono-ARTs, thus emerging as dual mono-ART inhibitors. Compounds 8a, 8h and 8l were also able to enter cells and rescue cells from apoptosis. Our work sheds more light on inhibitor development against mono-ARTs and identifies chemical probes to study the cellular roles of PARP10 and PARP15.
中文翻译:

强效 2,3-dihydrophthalazine-1,4-dione 衍生物作为单 ADP-核糖基转移酶 PARP10 和 PARP15 的双重抑制剂
虽然产生聚-ADP-核糖链的聚-ARTs,PARP1和2以及TNKS1和2已被广泛表征,但对单-ADP-核糖基化单-ARTs的病理生理学作用知之甚少,部分原因是缺乏选择性抑制剂。在这种情况下,我们专注于开发单 ART PARP10 抑制剂,已知其过表达会诱导细胞死亡。从 OUL35 ( 1 ) 及其 4-(苄氧基)苯甲酰胺衍生物 ( 2 ) 开始,我们在此报告了新类似物的设计和合成,其中环丁基衍生物3c最有效地从 PARP10 诱导的细胞凋亡中拯救细胞。最重要的是,我们还确定了 2,3-dihydrophthalazine-1,4-dione 作为一种新的合适的烟酰胺模拟 PARP10 抑制剂支架。当用环烷基 ( 8a-c )、邻氟苯基 ( 8h ) 和噻吩 ( 8l ) 环对其进行官能化时,获得了 130-160 nM 范围内的 IC 50值,使其成为迄今为止报道的最有效的 PARP10 抑制剂。这些化合物还以低微摩尔 IC 50 s抑制 PARP15 ,但没有其他测试的多和单 ARTs,因此作为双单 ART 抑制剂出现。化合物8a、8h和8l还能够进入细胞并拯救细胞免于凋亡。我们的工作揭示了针对单一 ART 的抑制剂开发,并确定了化学探针以研究 PARP10 和 PARP15 的细胞作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号