Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2022-04-19 , DOI: 10.1016/j.cclet.2022.04.026
Caocao Sun 1 , Guoyin Yin 1
|
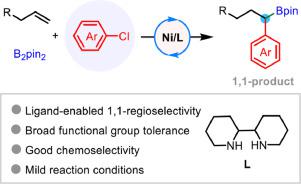
Difunctionalization of alkenes have developed into an important type of reactions for rapidly and efficiently assemble complex molecules. While extensive advancements have been achieved by the assistance of transition metal catalysis, the employment of cheap, abundant aryl chlorides as coupling partner is still a challenging task in this field. Herein, we report our first achievement in 1,1-difunctionalization of alkenes with aryl chlorides as coupling partners. The success is predominantly ascribed to the judicious selection of 1,2-diamine ligand. This study provides an efficient protocol for the synthesis of secondary benzyl boronates from easily accessible feedstock chemicals. Furthermore, the distinguished features of this method include excellent 1,1-regio- and chemoselectivity, good functional group tolerance and easily-operational catalytic reaction conditions.
中文翻译:

将芳基氯化物整合到镍催化的烯烃 1,1-双官能化中
烯烃的双官能化已发展成为快速有效地组装复杂分子的重要反应类型。虽然在过渡金属催化的帮助下取得了广泛的进展,但使用廉价、丰富的芳基氯化物作为偶联伙伴仍然是该领域的一项具有挑战性的任务。在这里,我们报告了我们在以芳基氯化物作为偶联伙伴的烯烃的 1,1-双官能化方面的第一项成就。成功主要归功于对 1,2-二胺配体的明智选择。本研究为从易于获取的原料化学品合成次硼酸苄酯提供了一种有效的方案。此外,该方法的显着特点包括出色的 1,1-区域和化学选择性,

































 京公网安备 11010802027423号
京公网安备 11010802027423号