Tetrahedron Letters ( IF 1.5 ) Pub Date : 2022-04-18 , DOI: 10.1016/j.tetlet.2022.153795 Scott T. Shreiber 1 , Gabriella I. Puchall 1 , David A. Vicic 1
|
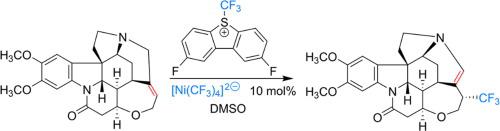
The homoleptic trifluoromethyl nickel complex [Ni(CF3)4]2− catalyzes a stereoselective trifluoromethylation of brucine with Umemoto II reagent. The trifluoromethylation process proceeds with concomitant isomerization of its alkenyl double bond leading to a trifluoromethylated neobrucine-like derivative. A minor difunctionalization product was also detected from the reaction mixture, consistent with a radical addition process that is occurring in parallel. Stoichiometric reactions between the nickel(II) catalyst precursor and brucine led to no reaction. However, a stochiometric reaction with a high-valent nickel(IV) complex formed trifluoromethylated neobrucine, implicating its intermediacy.
中文翻译:

使用均配镍催化剂 [Ni(CF3)4]2- 将马钱子碱转化为三氟甲基新马钱子碱
均配三氟甲基镍络合物 [Ni(CF 3 ) 4 ] 2-用 Umemoto II 试剂催化马钱子碱的立体选择性三氟甲基化。三氟甲基化过程伴随其烯基双键的异构化而进行,产生三氟甲基化的新布鲁辛样衍生物。还从反应混合物中检测到少量双官能化产物,这与平行发生的自由基加成过程一致。镍 (II) 催化剂前体和马钱子碱之间的化学计量反应没有导致反应。然而,与高价镍 (IV) 配合物的化学计量反应形成了三氟甲基化的新布鲁辛,暗示了它的中间体。






























 京公网安备 11010802027423号
京公网安备 11010802027423号